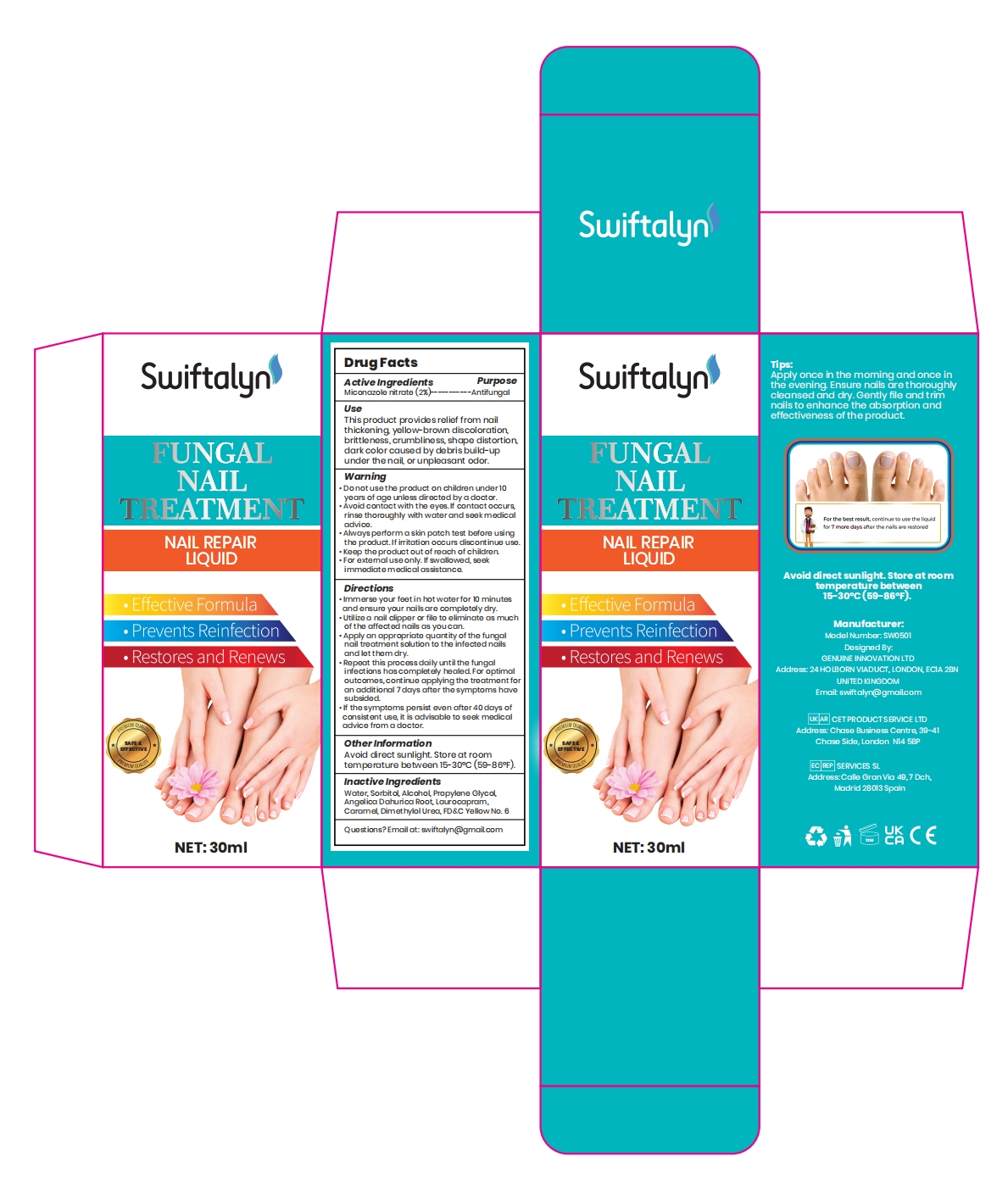
Label: SWIFTALYN FUNGAL NAIL TREATMENT- fungal nail treatment liquid
- NDC Code(s): 84048-002-01
- Packager: Shenzhen Peruidaishi Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
-
Warnings
Donotusethe product on children under10years of age unlass directed by a doctor.*Avoid contact with the eyes.if contact occursrinse thoroughly with waterand seek medicaladvica.
. Ahvays perform a s kin patch test bafore usingthe product.lf irritation occurs discontinue use..Kaep the product out of reach ofchildren.for axtemal use only. if swvallowed, saekimmediate medical assistanca - Do not use
- When Using
- Stop Use
- Keep Oot Of Reach Of Children
-
Directions
lmmerse your feat in hot water for 10 minutesand ensure your nails are completely dry.. Utilize a nail clipper or file to eliminate as muchof the affected nails as you can..Apply an appropriate quantity of the fungalnail treatment solution to the infected nailsand let them dry.
. Repeat this process daily until the fungalinfections hascompletety healed.for optimaloutcomes,continue applying the treatment foran additional 7days after the symptoms havesubsided
.if the symptoms parsist evan after 40 days ofconsistent use, it is advisable to seok medicaladvice from a doctor - Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SWIFTALYN FUNGAL NAIL TREATMENT
fungal nail treatment liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84048-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength SORBITOL (UNII: 506T60A25R) ANGELICA DAHURICA ROOT (UNII: 1V63N2S972) OXYMETHUREA (UNII: N68H97CAWG) ALCOHOL (UNII: 3K9958V90M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) LAUROCAPRAM (UNII: 1F3X9DRV9X) CARAMEL (UNII: T9D99G2B1R) WATER (UNII: 059QF0KO0R) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84048-002-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/24/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M029 01/24/2024 Labeler - Shenzhen Peruidaishi Technology Co., Ltd. (706728459) Establishment Name Address ID/FEI Business Operations Shenzhen Peruidaishi Technology Co., Ltd. 706728459 label(84048-002) , manufacture(84048-002)