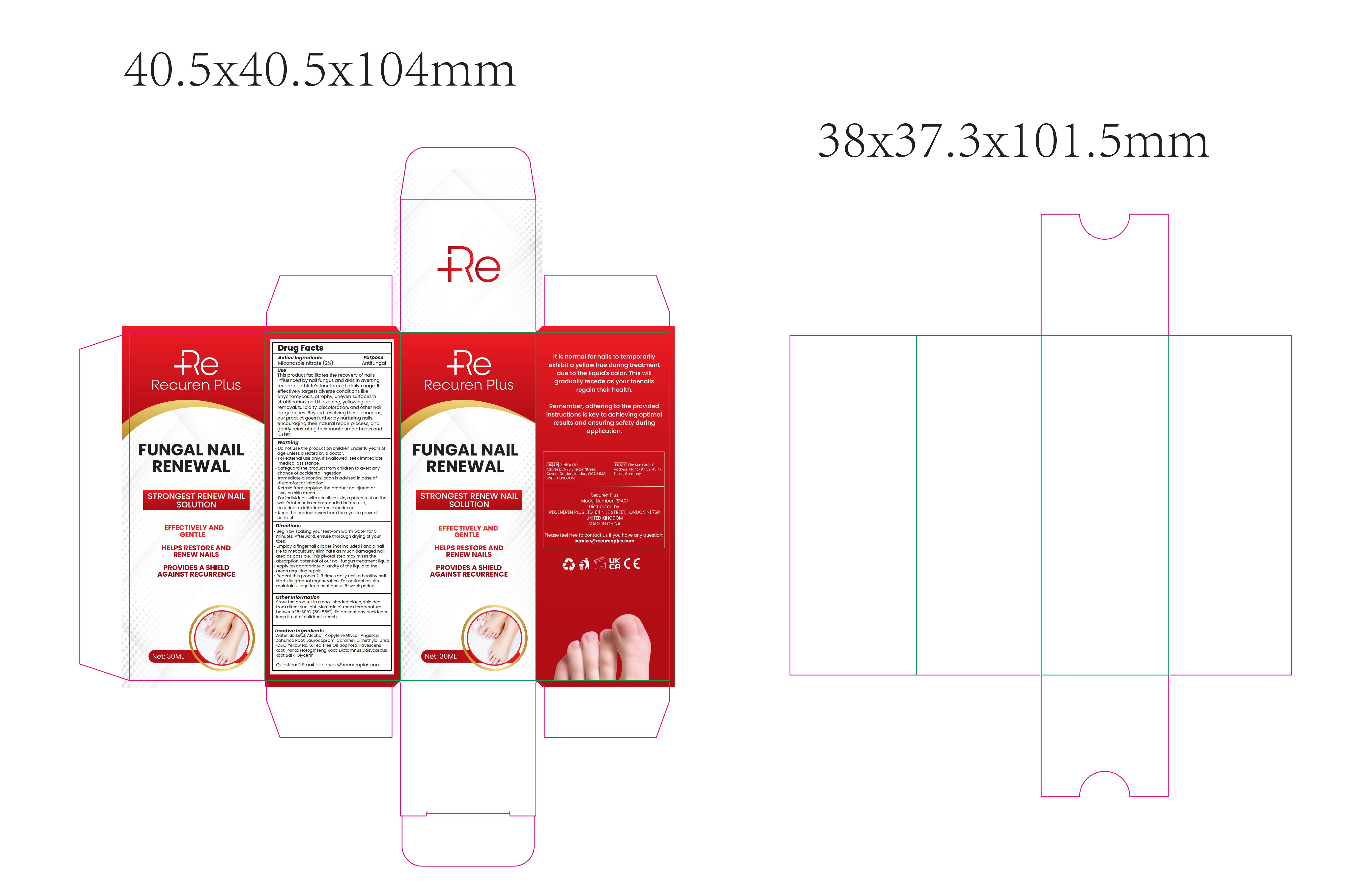
Label: RECUREN PLUS FUNGAL NAIL RENEWAL- fungal nail renewal liquid
- NDC Code(s): 84048-001-01
- Packager: Shenzhen Peruidaishi Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
-
Use
This product facilitates the recovery of nailsinfluenced by nail fungus and aids in avertingrecurrent athlete's foor through daily usage.lteffectively targets diverse conditions likeomychomycosis, atrophy, uneven surfacesmstratification, nail thickening, yellowing, nailremoval, turbidity, discoloration, and other nailirregulartes.Beyondresoling these concerns,our product goes further by nurturing nails,encouraging their natural repair process, andgently reinstating theirinnate smoothness andluster.
-
Warnings
* Do not use the product on children under 10 years ofage unless directed by a doctor.
*Forexternal use only. if swallowed, seek immediatemedical assistance.
*Safeguard the product from children to avert anychance of accidentalingestion..lmmediate discontinuation is advised in case of
discomfort or irritation.* Refrain from applying the product on injured orswollen skin areas.
*Forindividuals with sensitive skin, a patch test on thewrist's interior isrecommended before use,ensuring anirritation-free experience.
*Keep the product away from the eyes to preventcontact.
- Do not use
- When Using
- Stop Use
- Keep Oot Of Reach Of Children
-
Directions
Begin by soaking your feetvom warm water for 5minutes. Afterward, ensure thorough drying of yourto es.
Emnploy a fingemail clipper (not included) and a nailfile to meticulously eliminate as much damaged nailarea as possible.This pivotalstep maximizes theabsorption potential of our nail fungus treatment liquidApply an appropriate quantity of the liquid to theareas requiring repair.Repeat this proces 2-3 times daily until a healthy nailstarts its gradual regeneration, For optimal results,maintain usage for a continuous 6-week period. - Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RECUREN PLUS FUNGAL NAIL RENEWAL
fungal nail renewal liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84048-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MICONAZOLE NITRATE (UNII: VW4H1CYW1K) (MICONAZOLE - UNII:7NNO0D7S5M) MICONAZOLE NITRATE 2 g in 100 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) LAUROCAPRAM (UNII: 1F3X9DRV9X) CARAMEL (UNII: T9D99G2B1R) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) OXYMETHUREA (UNII: N68H97CAWG) SORBITOL (UNII: 506T60A25R) ALCOHOL (UNII: 3K9958V90M) ANGELICA DAHURICA ROOT (UNII: 1V63N2S972) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84048-001-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/24/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 01/24/2024 Labeler - Shenzhen Peruidaishi Technology Co., Ltd. (706728459) Establishment Name Address ID/FEI Business Operations Shenzhen Peruidaishi Technology Co., Ltd. 706728459 manufacture(84048-001) , label(84048-001)