Label: RITE-AID GLYCERIN LAXATIVE- glycerin suppository suppository
- NDC Code(s): 11822-9100-0, 11822-9100-5
- Packager: RITE AID CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 24, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
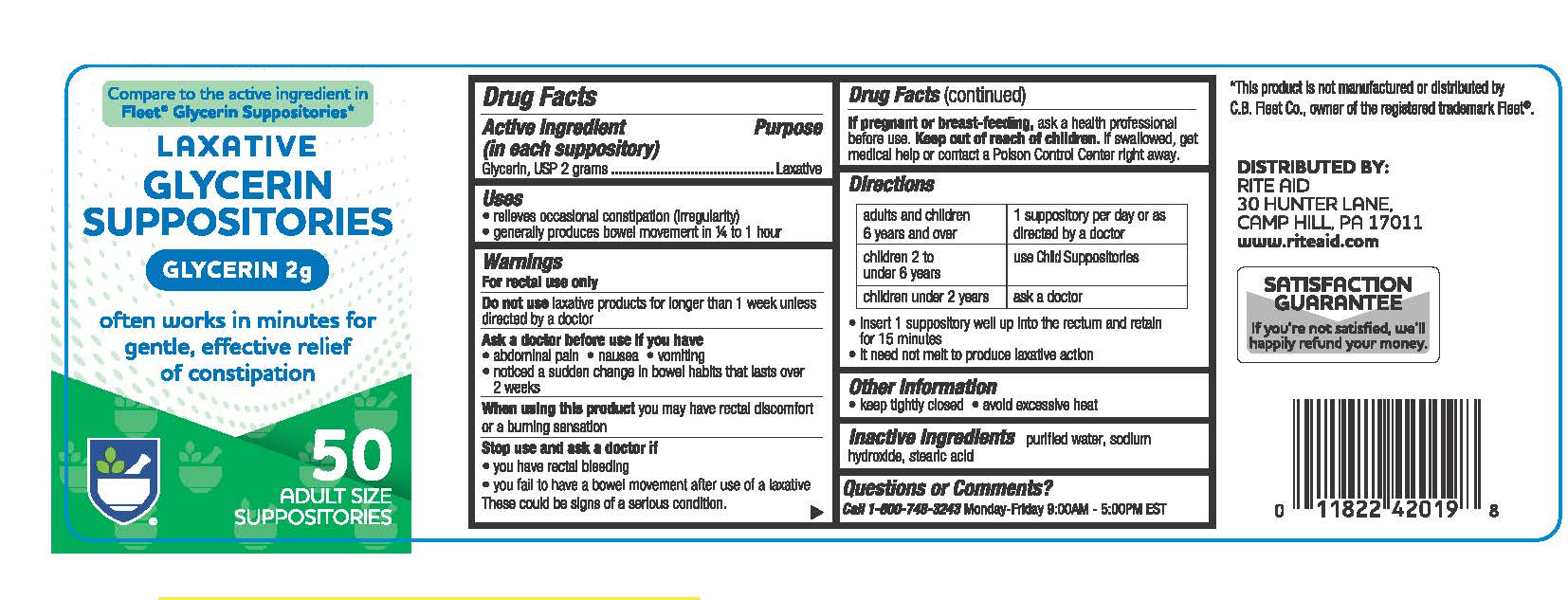
- Active Ingredient
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
- Rite-Aid® Glycerin Suppositories | 50 Count in a Jar
- Rite-Aid® Glycerin Suppositories | 100 Count in a Jar
-
INGREDIENTS AND APPEARANCE
RITE-AID GLYCERIN LAXATIVE
glycerin suppository suppositoryProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-9100 Route of Administration RECTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 2 g Inactive Ingredients Ingredient Name Strength STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM HYDROXIDE (UNII: 55X04QC32I) WATER (UNII: 059QF0KO0R) Product Characteristics Color white (CLEAR) Score Shape BULLET Size 32mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-9100-5 50 in 1 JAR; Type 0: Not a Combination Product 01/01/2024 2 NDC:11822-9100-0 100 in 1 JAR; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 01/01/2012 Labeler - RITE AID CORPORATION (014578892) Registrant - Unipack LLC (116015769) Establishment Name Address ID/FEI Business Operations Unipack LLC 009248480 manufacture(11822-9100)