Label: DIANEAL LOW CALCIUM WITH DEXTROSE- sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solution
-
NDC Code(s):
0941-0686-01,
0941-0686-08,
0941-0690-01,
0941-0690-08, view more0941-0694-01, 0941-0694-08
- Packager: Baxter Healthcare Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Unapproved drug for use in drug shortage
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 7, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Health Care Provider Letter
-
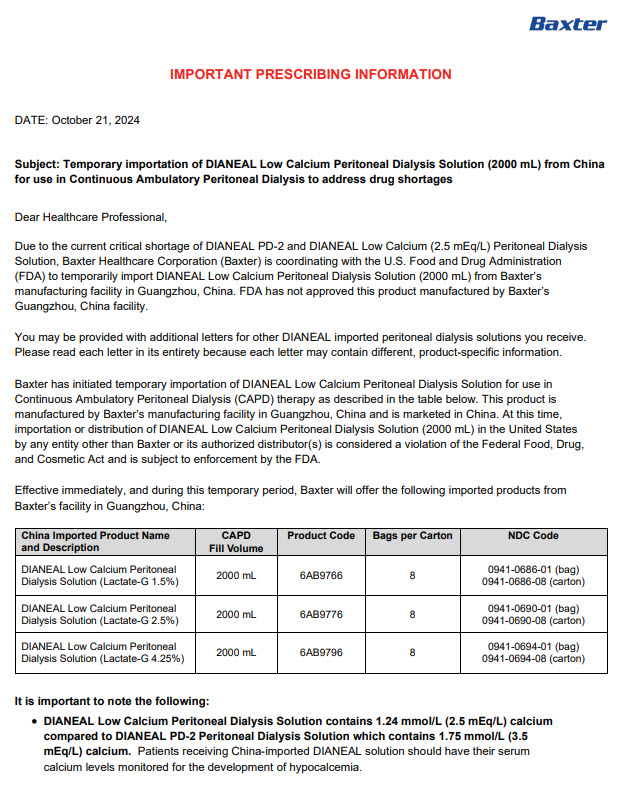
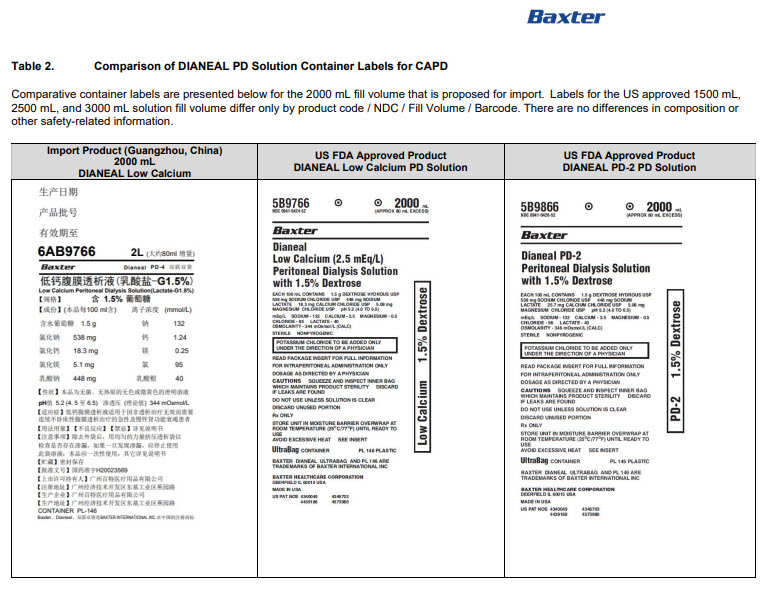
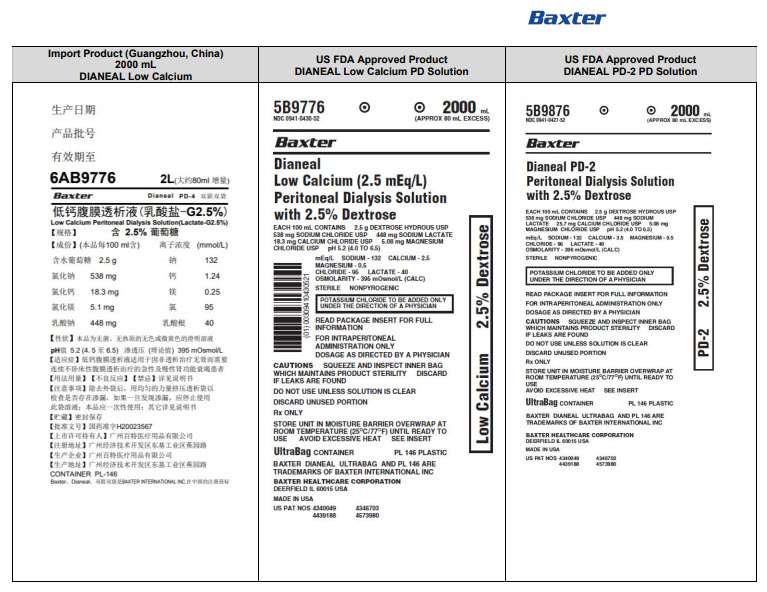
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
Manufacturing Date
Lot Number
EXP
6AB9766 2L (APPROX 80ml EXCESS)
BaxterLogo Dianeal PD-4 Ultrabag
Low Calcium Peritoneal Dialysis Solution (Lactate-G1.5%)
[Strengths]Containing 1.5% glucose
[Ingredients](Each 100 ml contains) Ionic concentration(mmol/L)Dextrose Hydrous
1.5 g
Sodium
132
Sodium Chloride
538mg
Calcium
1.24
Calcium Chloride
18.3mg
Magnesium
0.25
Magnesium Chloride
5.1mg
Chloride
95
Sodium Lactate
448mg
Lactate
40
[Characters]This product is a sterile, Nonpyrogen, Colorless
or slightly yellow, Clear solution, pH 5.2 (4.5 to 6.5)Calculated osmolarity(theoretical value) 344 mOsmol/L
[Indications]Low-calcium peritoneal dialysis solutions are
indicated for use in acute and chronic renal failure patients
being maintained on continuous ambulatory peritoneal dialysis
when nondialytic medical therapy is judged to be inadequate[Usage and Dosage] [Adverse Reactions]
[Contraindications] See instructions for details[Precautions]After removing the outer bag, squeeze the
dialysis bag with uniform force to check for leakage,
If leakage is found, stop using the solution;
This product should be used once;
For other details, please refer to the instructions[Storage]Keep sealed
[Approval License Number]GYZZ H20023569
[Approval License Holder]BAXTER
HEALTHCARE(GUANGZHOU) Co., Ltd[Legal Address]Jiaoyuan Road, Dongji industrial District,
GETDD, Guangzhou, P.R. China[Manufacturer]BAXTER HEALTHCARE(GUANGZHOU) Co., Ltd
[Manufacturing Address]Jiaoyuan Road, Dongji industrial
District GETDD,Guangzhou, P.R. ChinaCONTAINER PL-146
Baxter, Dianeal, Ultrabag are registered trademarks of BAXTER INTERNATIONAL INC. in ChinaLow Calcium Peritoneal Dialysis Solution (Lactate-G1.5%)
[Specification] Contain 1.5% Dextrose 6AB9766
Lot G00000000 2LX 8 Bags
Manu. Date 0000-00-00 PD-4 UltraBag
Valid in 0000-00
Barcode
6 9 3 7 7 6 5 3 0 0 2 3 3 >S/N 0000
Manufacturing Date
Lot Number
EXP
6AB9776 2L (APPROX 80ml EXCESS)
BaxterLogo Dianeal PD-4 Ultrabag
Low Calcium Peritoneal Dialysis Solution (Lactate-G2.5%)
[Strengths]Containing 2.5% glucose
[Ingredients](Each 100 ml contains) Ionic concentration(mmol/L)Dextrose Hydrous
2.5 g
Sodium
132
Sodium Chloride
538mg
Calcium
1.24
Calcium Chloride
18.3mg
Magnesium
0.25
Magnesium Chloride
5.1mg
Chloride
95
Sodium Lactate
448mg
Lactate
40
[Characters]This product is a sterile, Nonpyrogen, Colorless
or slightly yellow, Clear solution, pH 5.2 (4.5 to 6.5)Calculated osmolarity(theoretical value) 395 mOsmol/L
[Indications]Low-calcium peritoneal dialysis solutions are
indicated for use in acute and chronic renal failure patients
being maintained on continuous ambulatory peritoneal dialysis
when nondialytic medical therapy is judged to be inadequate[Usage and Dosage] [Adverse Reactions]
[Contraindications] See instructions for details[Precautions]After removing the outer bag, squeeze the
dialysis bag with uniform force to check for leakage,
If leakage is found, stop using the solution;
This product should be used once;
For other details, please refer to the instructions[Storage]Keep sealed
[Approval License Number]GYZZ H20023567
[Approval License Holder]BAXTER
HEALTHCARE(GUANGZHOU) Co., Ltd[Legal Address]Jiaoyuan Road, Dongji industrial District,
GETDD, Guangzhou, P.R. China[Manufacturer]BAXTER HEALTHCARE(GUANGZHOU) Co., Ltd
[Manufacturing Address]Jiaoyuan Road, Dongji industrial
District GETDD,Guangzhou, P.R. ChinaCONTAINER PL-146
Baxter, Dianeal, Ultrabag are registered trademarks of BAXTER INTERNATIONAL INC. in ChinaLow Calcium Peritoneal Dialysis Solution (Lactate-G2.5%)
[Specification] Contain 1.5% Dextrose 6AB9776
Lot G00000000 2LX 8 Bags
Manu. Date 0000-00-00 PD-4 UltraBag
Valid in 0000-00
Barcode
6 9 3 7 7 6 5 3 0 0 2 4 0 >S/N 0000
Manufacturing Date
Lot Number
EXP
6AB9796 2L (APPROX 80ml EXCESS)
BaxterLogo Dianeal PD-4 Ultrabag
Low Calcium Peritoneal Dialysis Solution (Lactate-G4.25%)
[Strengths]Containing 4.25% glucose
[Ingredients](Each 100 ml contains) Ionic concentration(mmol/L)Dextrose Hydrous
4.25 g
Sodium
132
Sodium Chloride
538mg
Calcium
1.24
Calcium Chloride
18.3mg
Magnesium
0.25
Magnesium Chloride
5.1mg
Chloride
95
Sodium Lactate
448mg
Lactate
40
[Characters]This product is a sterile, Nonpyrogen, Colorless
or slightly yellow, Clear solution, pH 5.2 (4.5 to 6.5)Calculated osmolarity(theoretical value) 483 mOsmol/L
[Indications]Low-calcium peritoneal dialysis solutions are
indicated for use in acute and chronic renal failure patients
being maintained on continuous ambulatory peritoneal dialysis
when nondialytic medical therapy is judged to be inadequate[Usage and Dosage] [Adverse Reactions]
[Contraindications] See instructions for details[Precautions]After removing the outer bag, squeeze the
dialysis bag with uniform force to check for leakage,
If leakage is found, stop using the solution;
This product should be used once;
For other details, please refer to the instructions[Storage]Keep sealed
[Approval License Number]GYZZ H20023568
[Approval License Holder]BAXTER
HEALTHCARE(GUANGZHOU) Co., Ltd[Legal Address]Jiaoyuan Road, Dongji industrial District,
GETDD, Guangzhou, P.R. China[Manufacturer]BAXTER HEALTHCARE(GUANGZHOU) Co., Ltd
[Manufacturing Address]Jiaoyuan Road, Dongji industrial
District GETDD,Guangzhou, P.R. ChinaCONTAINER PL-146
Baxter, Dianeal, Ultrabag are registered trademarks of BAXTER INTERNATIONAL INC. in ChinaLow Calcium Peritoneal Dialysis Solution (Lactate-G4.25%)
[Specification] Contain 1.5% Dextrose 6AB9796
Lot G00000000 2LX 8 Bags
Manu. Date 0000-00-00 PD-4 UltraBag
Valid in 0000-00
Barcode
6 9 3 7 7 6 5 3 0 0 2 5 7 >S/N 0000
-
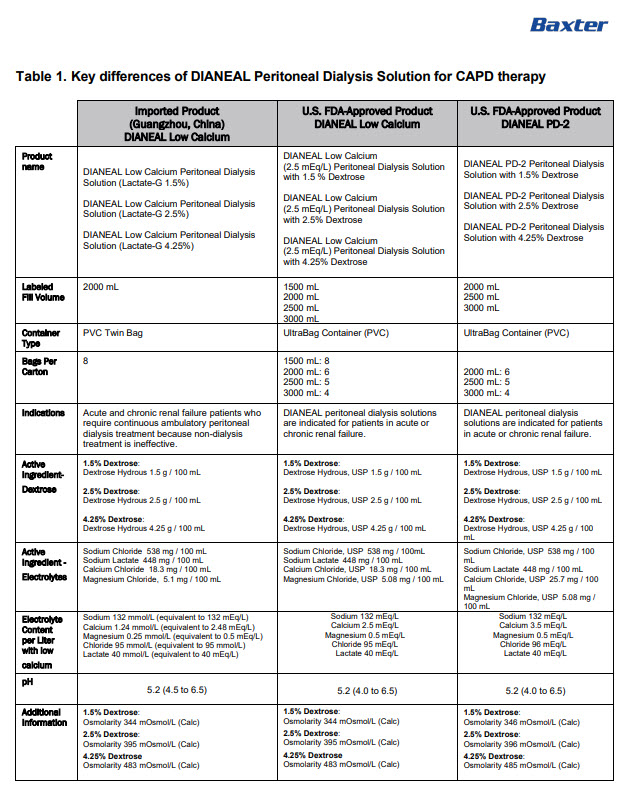
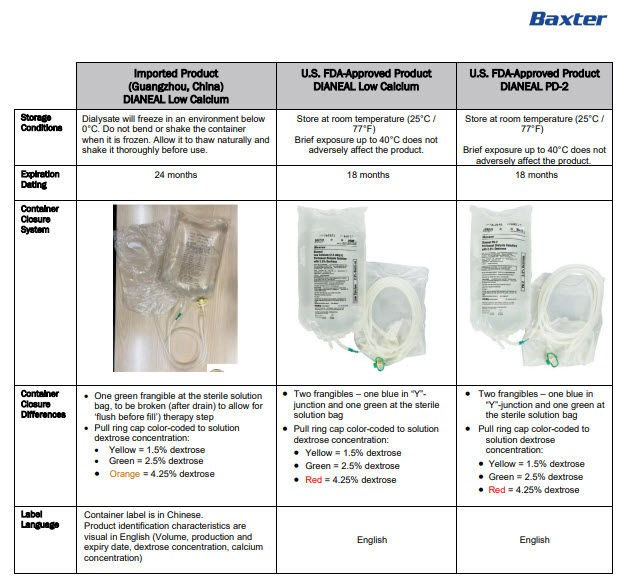
INGREDIENTS AND APPEARANCE
DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0686 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 1.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.1 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0686-08 8 in 1 CARTON 11/01/2024 1 NDC:0941-0686-01 2000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/01/2024 DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0690 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 2.5 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.1 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0690-08 8 in 1 CARTON 11/01/2024 1 NDC:0941-0690-01 2000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/01/2024 DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:0941-0694 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 4.25 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.1 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0941-0694-08 8 in 1 CARTON 11/01/2024 1 NDC:0941-0694-01 2000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 11/01/2024 Labeler - Baxter Healthcare Corporation (005083209) Establishment Name Address ID/FEI Business Operations Baxter Healthcare (Guangzhou) Co., Ltd 421040114 analysis(0941-0686, 0941-0690, 0941-0694) , label(0941-0686, 0941-0690, 0941-0694) , manufacture(0941-0686, 0941-0690, 0941-0694) , pack(0941-0686, 0941-0690, 0941-0694) , sterilize(0941-0686, 0941-0690, 0941-0694)