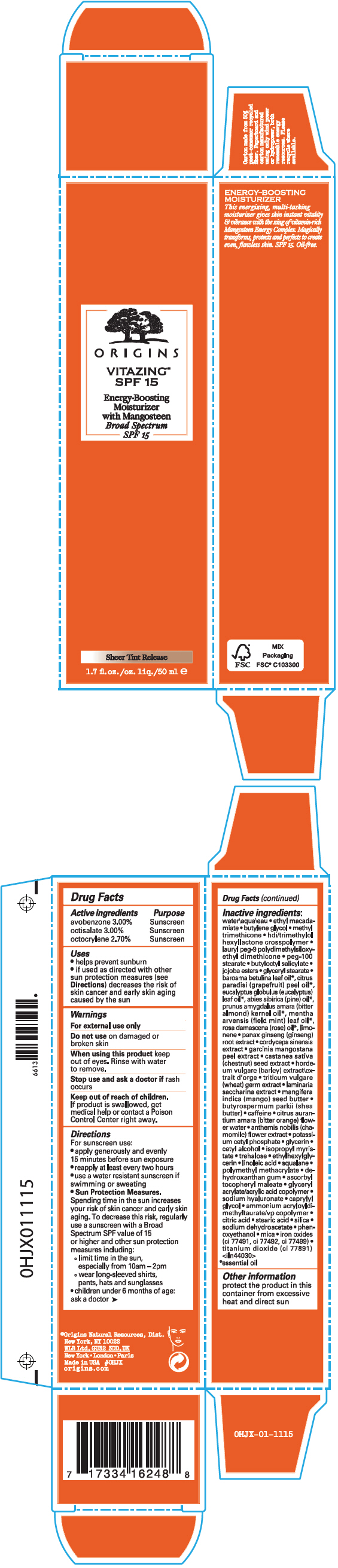
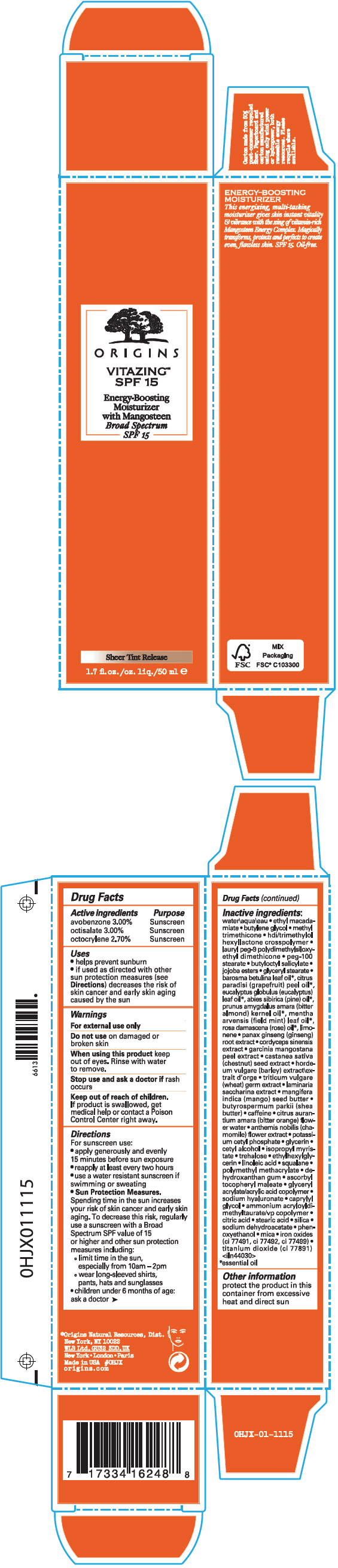
Label: VITAZING SPF 15 ENERGY BOOSTING MOISTURIZER WITH MANGOSTEEN BROAD SPECTRUM SPF 15- avobenzone, octisalate, and octocrylene cream
- NDC Code(s): 59427-013-01
- Packager: ORIGINS NATURAL RESOURCES INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 5, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply generously and evenly 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10am – 2pm
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqua\eau • ethyl macadamiate • butylene glycol • methyl trimethicone • hdi/trimethylol hexyllactone crosspolymer • lauryl peg-9 polydimethylsiloxyethyl dimethicone • peg-100 stearate • butyloctyl salicylate • jojoba esters • glyceryl stearate • barosma betulina leaf oil 1, citrus paradisi (grapefruit) peel oil 1, eucalyptus globulus (eucalyptus) leaf oil 1, abies sibirica (pine) oil 1, prunus amygdalus amara (bitter almond) kernel oil 1, mentha arvensis (field mint) leaf oil 1, rosa damascena (rose) oil 1, limonene • panax ginseng (ginseng) root extract • cordyceps sinensis extract • garcinia mangostana peel extract • castanea sativa (chestnut) seed extract • hordeum vulgare (barley) extract\extrait d'orge • triticum vulgare (wheat) germ extract • laminaria saccharina extract • mangifera indica (mango) seed butter • butyrospermum parkii (shea butter) • caffeine • citrus aurantium amara (bitter orange) flower water • anthemis nobilis (chamomile) flower extract • potassium cetyl phosphate • glycerin • cetyl alcohol • isopropyl myristate • trehalose • ethylhexylglycerin • linoleic acid • squalane • polymethyl methacrylate • dehydroxanthan gum • ascorbyl tocopheryl maleate • glyceryl acrylate/acrylic acid copolymer • sodium hyaluronate • caprylyl glycol • ammonium acryloyldimethyltaurate/vp copolymer • citric acid • stearic acid • silica • sodium dehydroacetate • phenoxyethanol • mica • iron oxides (ci 77491, ci 77492, ci 77499) • titanium dioxide (ci 77891) <iln44030>
- 1
- essential oil
- Other information
- PRINCIPAL DISPLAY PANEL - 50 ml Tube Carton
-
INGREDIENTS AND APPEARANCE
VITAZING SPF 15 ENERGY BOOSTING MOISTURIZER WITH MANGOSTEEN BROAD SPECTRUM SPF 15
avobenzone, octisalate, and octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59427-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 30 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 27 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ETHYL MACADAMIATE (UNII: ANA2NCS6V1) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) LAURYL PEG-9 POLYDIMETHYLSILOXYETHYL DIMETHICONE (UNII: 25G622K2RA) PEG-100 STEARATE (UNII: YD01N1999R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) AGATHOSMA BETULINA LEAF OIL (UNII: KOS935A04V) GRAPEFRUIT OIL (UNII: YR377U58W9) EUCALYPTUS OIL (UNII: 2R04ONI662) WHITE PINE OIL (UNII: HA5CX6676U) BITTER ALMOND OIL (UNII: 6TQK77W0EX) MENTHA ARVENSIS LEAF OIL (UNII: 1AEY1M553N) ROSE OIL (UNII: WUB68Y35M7) ASIAN GINSENG (UNII: CUQ3A77YXI) BARLEY (UNII: 5PWM7YLI7R) WHEAT (UNII: 4J2I0SN84Y) MANGIFERA INDICA SEED BUTTER (UNII: 4OXD9M35X2) SHEA BUTTER (UNII: K49155WL9Y) CAFFEINE (UNII: 3G6A5W338E) BITTER ORANGE (UNII: DQD16J2B5O) CHAMOMILE (UNII: FGL3685T2X) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) GLYCERIN (UNII: PDC6A3C0OX) CETYL ALCOHOL (UNII: 936JST6JCN) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) TREHALOSE (UNII: B8WCK70T7I) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) LINOLEIC ACID (UNII: 9KJL21T0QJ) SQUALANE (UNII: GW89575KF9) POLY(METHYL METHACRYLATE; 450000 MW) (UNII: Z47NNT4J11) DEHYDROXANTHAN GUM (UNII: 63ZP7I1BQO) ASCORBYL TOCOPHERYL MALEATE (UNII: D2G6259XR5) HYALURONATE SODIUM (UNII: YSE9PPT4TH) CAPRYLYL GLYCOL (UNII: 00YIU5438U) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) STEARIC ACID (UNII: 4ELV7Z65AP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59427-013-01 1 in 1 CARTON 01/01/2017 1 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final M020 01/01/2017 Labeler - ORIGINS NATURAL RESOURCES INC. (611716283) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(59427-013) , pack(59427-013) , label(59427-013)