Label: UREA cream
- NDC Code(s): 24470-939-01, 24470-939-03, 24470-939-07
- Packager: Cintex Services, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated January 23, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Rx Only
FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
DESCRIPTION:
This product is a keratolytic emollient which is a gentle, yet potent, tissue softener for nails and/or skin.
Each gram contains 400 mg of urea in a vehicle consisting of: carbomer, cetyl alcohol, dimethyl isosorbide, glyceryl stearate, mineral oil, petrolatum, propylene glycol, purified water, sodium hydroxide and xanthan gum.
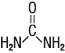
Urea is a diamide of carbonic acid with the following chemical structure:
- CLINICAL PHARMACOLOGY:
-
INDICATIONS:
For debridement and promotion of normal healing of hyperkeratotic surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris or eschar. Urea is useful for the treatment of hyperkeratotic conditions such as dry, rough skin, dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis pilaris, keratosis palmaris, keratoderma, corns and calluses, as well as damaged, ingrown and devitalized nails.
- CONTRAINDICATIONS:
-
WARNINGS AND PRECAUTIONS
WARNINGS: KEEP OUT OF REACH OF CHILDREN.
PRECAUTIONS: FOR EXTERNAL USE ONLY. NOT FOR OPHTHALMIC USE.
General: This product is to be used as directed by a physician and should not be used to treat any condition other than that for which it was prescribed. If redness or irritation occurs, discontinue use and consult a physician.
Information for Patients: Patients should discontinue the use of this product if the condition becomes worse or if a rash develops in the area being treated or elsewhere. Avoid contact with eyes, lips and mucous membranes.
Carcinogenesis, Mutagenesis and Impairment of Fertility: Long-term animal studies for carcinogenic potential have not been performed on this product to date. Studies on reproduction and fertility also have not been performed.
Pregnancy:Category C. Animal reproduction studies have not been conducted with this product. It is also not known whether this product can affect reproduction capacity or cause fetal harm when administered to a pregnant woman. This product should be used by a pregnant woman only if clearly needed or when potential benefits outweigh potential hazards to the fetus.
Nursing Mothers: It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when this product is administered to a nursing woman.
- ADVERSE REACTIONS:
- DOSAGE AND ADMINISTRATION:
-
STORAGE:
Store at 20°C to 25°C (68°F to 77°F), excursions permitted between 15°C to 30°C (between 59°F to 86°F). Brief exposure to temperatures up to 40°C (104°F) may be tolerated provided the mean kinetic temperature does not exceed 25°C (77°F); however, such exposure should be minimized.
NOTICE: Protect from freezing and excessive heat. Keep container tightly closed.
-
HOW SUPPLIED:
1 oz. (28.35 g) bottles, NDC 24470-939-01
3 oz. (85 g) bottles, NDC 24470-939-03
7 oz. (198.4 g) bottles, NDC 24470-939-07
To report a serious adverse event or obtain product information, call 1-855-899-4237.
Manufactured for:
Cintex Services, LLC
9330 LBJ Freeway, Suite 900
Dallas, TX 75243
2300402 [00] Rev. 12/2023
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UREA
urea creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:24470-939 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength UREA (UNII: 8W8T17847W) (UREA - UNII:8W8T17847W) UREA 400 mg in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24470-939-01 1 in 1 CARTON 01/23/2024 1 28.35 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 2 NDC:24470-939-03 85 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/23/2024 3 NDC:24470-939-07 198.4 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 01/23/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/23/2024 Labeler - Cintex Services, LLC (078304114)






