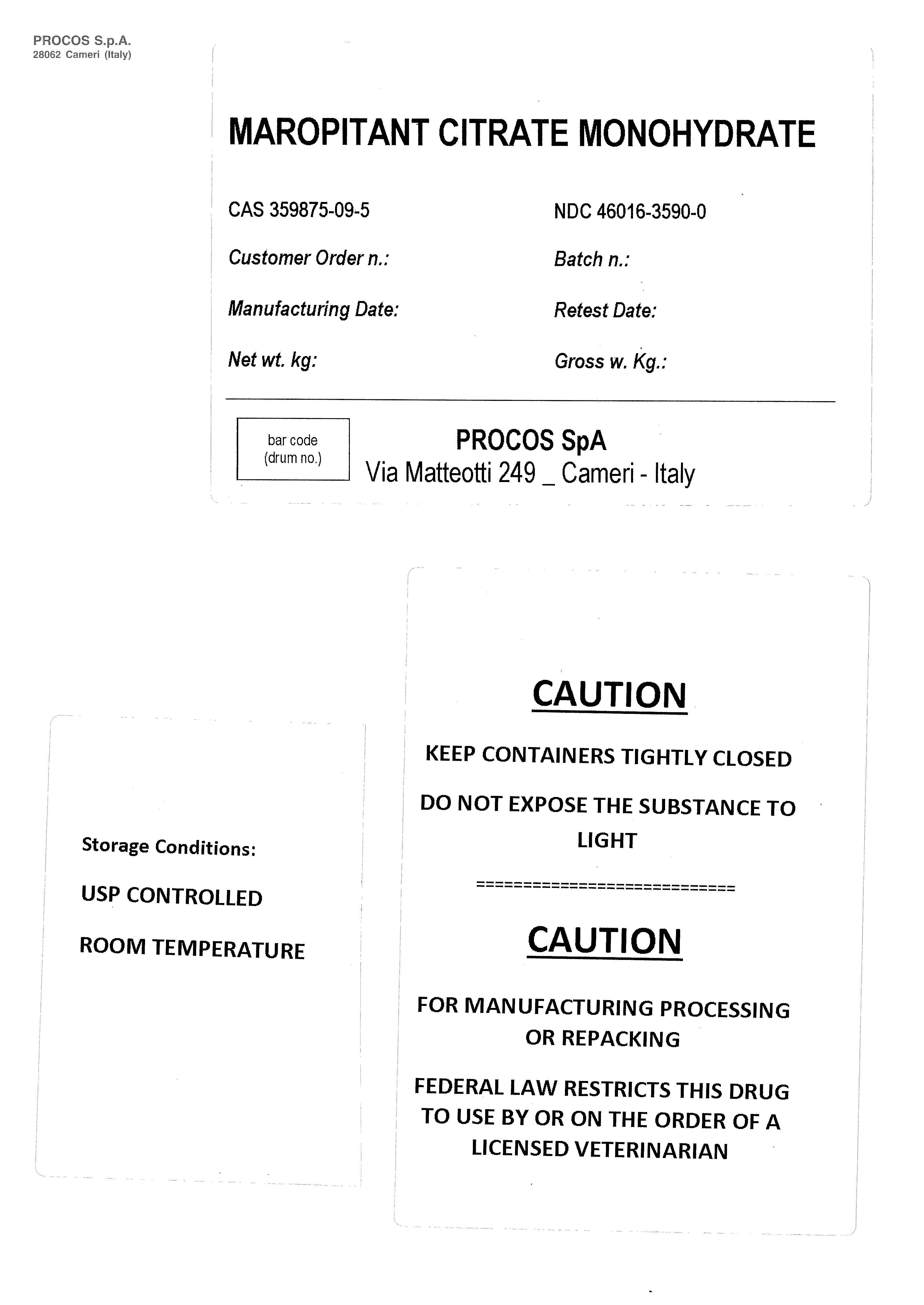
Label: MAROPITANT CITRATE MONOHYDRATE- maropitant citrate powder
- NDC Code(s): 46016-3590-0
- Packager: PROCOS SpA
- Category: BULK INGREDIENT - ANIMAL DRUG
- DEA Schedule: None
- Marketing Status: Bulk Ingredient For Animal Drug Compounding
Drug Label Information
Updated October 22, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
SPL UNCLASSIFIED SECTION
MAROPITANT CITRATE MONOHYDRATE
CAS 359875-09-5 NDC 46016-3590-0
Customer Order n.: Batch n:
Manufacturing Date: Retest Date:
Net wt. kg: Gross w. Kg:
CAUTION
KEEP CONTAINERS TIGHTLY CLOSED. DO NOT EXPOSE THE SUBSTANCE TO LIGHT
CAUTION
FOR MANUFACTURING PROCESSING OR REPACKING
FEDERAL LAW RESTRICTS THIS DRUG TO USE BY OR ON THE ORDER OF A LICENSED VETERINARIAN
Storage Conditions:
USP CONTROLLED
ROOM TEMPERATURE
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MAROPITANT CITRATE MONOHYDRATE
maropitant citrate powderProduct Information Product Type Item Code (Source) NDC:46016-3590 Route of Administration NOT APPLICABLE Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAROPITANT CITRATE (UNII: LXN6S3999X) (MAROPITANT - UNII:4XE2T9H4DH) MAROPITANT CITRATE 50 kg in 50 kg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46016-3590-0 50 kg in 1 CONTAINER Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date bulk ingredient for animal drug compounding 11/26/2018 Labeler - PROCOS SpA (439262429) Establishment Name Address ID/FEI Business Operations PROCOS SpA 439262429 api manufacture