Label: DIPHENHYDRAMINE HCL ORAL SOLUTION- diphenhydramine hcl oral solution
- NDC Code(s): 0904-7323-41, 0904-7323-70, 0904-7324-66, 0904-7324-72
- Packager: MAJOR® PHARMACEUTICALS
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 19, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient per 5 mL (1 Unit Dose)
- Active ingredient per 10 mL (1 Unit Dose)
- Purpose
- Uses
-
Warnings
Do not use
• to make a child sleepy
• with any other product containing diphenhydramine, even one used on skinAsk a doctor before use if the child has
• a breathing problem such as chronic bronchitis
• glaucoma -
Directions
For the 5 mL Unit-Dose:
• take every 4 to 6 hours, or as directed by a physician
• do not take more than 6 doses in 24 hours
age dose Children under 2 years of age Do not use Children 2 to under 6 years of age Do not use unless directed by a doctor Children 6 to under 12 years of age 5 mL (12.5 mg) to 10 mL (25 mg) -
Directions
For the 10 mL Unit-Dose:
• take every 4 to 6 hours, or as directed by a physician
• do not take more than 6 doses in 24 hoursage dose Children under 6 years of age Do not use Children 6 to under 12 years of age 10 mL (25 mg) Adults and children 12 years of age and over 10 mL (25 mg) to 20 mL (50 mg) - Other information
- SPL UNCLASSIFIED SECTION
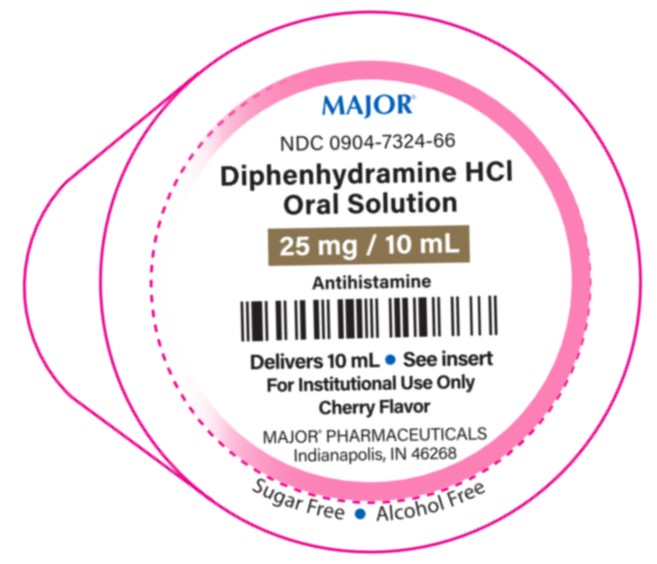
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HCL ORAL SOLUTION
diphenhydramine hcl oral solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-7323 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C RED NO. 40 (UNII: WZB9127XOA) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM CITRATE (UNII: 1Q73Q2JULR) SUCRALOSE (UNII: 96K6UQ3ZD4) WATER (UNII: 059QF0KO0R) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-7323-70 100 in 1 BOX, UNIT-DOSE 01/22/2024 1 NDC:0904-7323-41 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/22/2024 DIPHENHYDRAMINE HCL ORAL SOLUTION
diphenhydramine hcl oral solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-7324 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg in 10 mL Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C RED NO. 40 (UNII: WZB9127XOA) METHYLPARABEN (UNII: A2I8C7HI9T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM CITRATE (UNII: 1Q73Q2JULR) SUCRALOSE (UNII: 96K6UQ3ZD4) WATER (UNII: 059QF0KO0R) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-7324-72 100 in 1 BOX, UNIT-DOSE 01/22/2024 1 NDC:0904-7324-66 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 01/22/2024 Labeler - MAJOR® PHARMACEUTICALS (191427277)