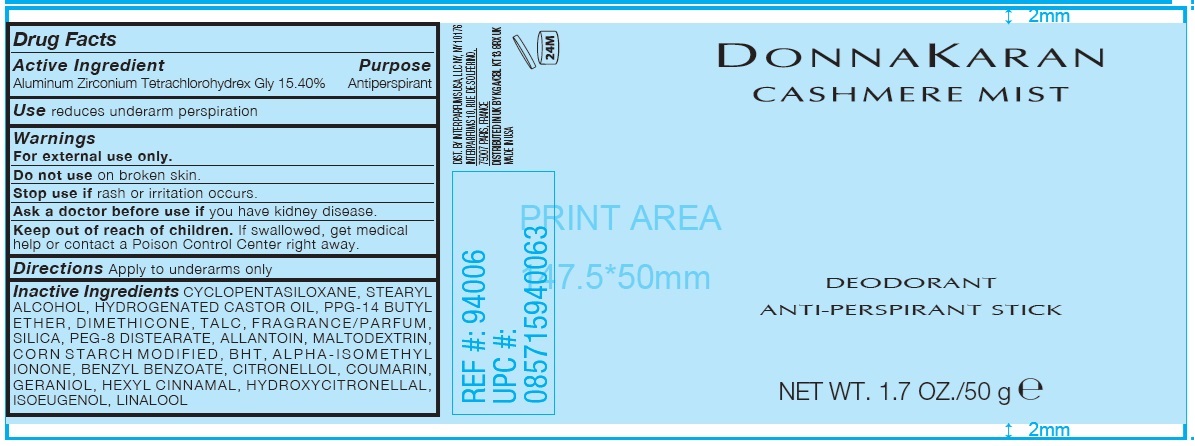
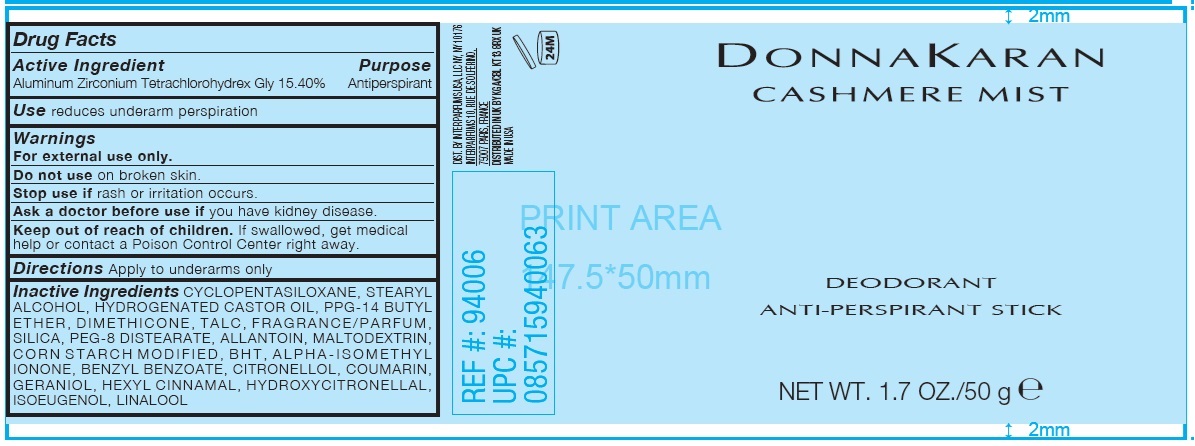
Label: DONNA KARAN CASHMERE MIST DEODORANT ANTI-PERSPIRANT- aluminum zirconium tetrachlorohydrex gly stick
- NDC Code(s): 84012-001-01, 84012-001-02
- Packager: Interparfums USA, LLC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 22, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
-
Inactive Ingredients
CYCLOPENTASILOXANE, STEARYL ALCOHOL, HYDROGENATED CASTOR OIL, PPG-14 BUTYL ETHER, DIMETHICONE, TALC, FRAGRANCE/PARFUM, SILICA, PEG-8 DISTEARATE, ALLANTOIN, MALTODEXTRIN, CORN STARCH MODIFIED, BHT, ALPHA-ISOMETHYL IONONE, BENZYL BENZOATE, CITRONELLOL, COUMARIN, GERANIOL, HEXYL CINNAMAL, HYDROXYCITRONELLAL, ISOEUGENOL, LINALOOL
- Product Packaging
-
INGREDIENTS AND APPEARANCE
DONNA KARAN CASHMERE MIST DEODORANT ANTI-PERSPIRANT
aluminum zirconium tetrachlorohydrex gly stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84012-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY (UNII: 8O386558JE) (ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY - UNII:8O386558JE) ALUMINUM ZIRCONIUM TETRACHLOROHYDREX GLY 154 mg in 1 g Inactive Ingredients Ingredient Name Strength CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) PPG-14 BUTYL ETHER (UNII: R199TJT95T) DIMETHICONE (UNII: 92RU3N3Y1O) TALC (UNII: 7SEV7J4R1U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) PEG-8 DISTEARATE (UNII: 7JNC8VN07M) ALLANTOIN (UNII: 344S277G0Z) MALTODEXTRIN (UNII: 7CVR7L4A2D) .ALPHA.-HEXYLCINNAMALDEHYDE (UNII: 7X6O37OK2I) HYDROXYCITRONELLAL (UNII: 8SQ0VA4YUR) LINALOOL, (+/-)- (UNII: D81QY6I88E) .BETA.-CITRONELLOL, (R)- (UNII: P01OUT964K) ISOMETHYL-.ALPHA.-IONONE (UNII: 9XP4LC555B) BENZYL BENZOATE (UNII: N863NB338G) COUMARIN (UNII: A4VZ22K1WT) GERANIOL (UNII: L837108USY) ISOEUGENOL (UNII: 5M0MWY797U) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84012-001-01 50 g in 1 CANISTER; Type 0: Not a Combination Product 04/08/2020 2 NDC:84012-001-02 3 in 1 CARTON 04/08/2020 2 50 g in 1 CANISTER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 04/08/2020 Labeler - Interparfums USA, LLC. (080820309)