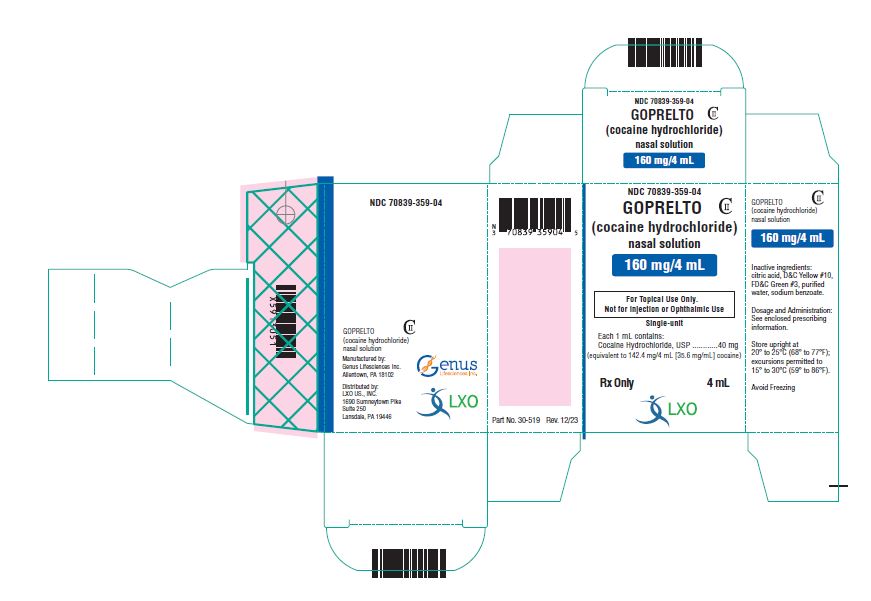
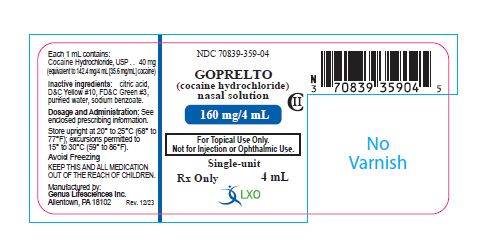
Label: GOPRELTO- cocaine hydrochloride solution
- NDC Code(s): 70839-359-04
- Packager: LXO US Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: CII
- Marketing Status: New Drug Application
Drug Label Information
Updated November 6, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GOPRELTO safely and effectively. See full prescribing information for GOPRELTO.
GOPRELTO (cocaine hydrochloride) nasal solution, for intranasal use, CII
Initial U.S. Approval: 2017WARNING: ABUSE AND DEPENDENCE
See full prescribing information for complete boxed warning.
CNS stimulants, including cocaine hydrochloride, have a high potential for abuse and dependence. (5.1)
INDICATIONS AND USAGE
GOPRELTO (cocaine hydrochloride) nasal solution is an ester local anesthetic indicated for the induction of local anesthesia of the mucous membranes when performing diagnostic procedures and surgeries on or through the nasal cavities in adults. ( 1)
DOSAGE AND ADMINISTRATION
• For intranasal use only. ( 2.1)
• Recommended dose: two pledgets, each containing 40 mg of cocaine hydrochloride,applied to each nasal cavity. ( 2.2)
• Do not apply to damaged nasal mucosa. ( 2.1)
• Preparation and Application:
– In a small container, soak four pledgets in the full contents (4 mL) of one bottle of GOPRELTO until the solution is fully absorbed. Each pledget absorbs 1 mL of solution,
equivalent to 40 mg cocaine hydrochloride. ( 2.2, 2.3)
– Following soaking, place two pledgets in each nasal cavity against the septum. ( 2.3)
– Leave pledgets in p lace for up to 20 minutes. ( 2.3)DOSAGE FORMS AND STRENGTHS
Nasal solution: 160 mg/4 mL (40 mg/mL or 4%) cocaine hydrochloride, equivalent to 142.4 mg/4 mL (35.6 mg/mL) cocaine, in a single-unit bottle. ( 3)
CONTRAINDICATIONS
Known hypersensitivity to cocaine hydrochloride, other ester-based anesthetics, or any other component of GOPRELTO. ( 4)
WARNINGS AND PRECAUTIONS
• Seizures: GOPRELTO may lower the convulsive threshold. Monitor patients for development of seizures. ( 5.2)
• Blood Pressure and Heart Rate Increases: Monitor vital signs, including heart rate and rhythm, in patients after receiving GOPRELTO. Avoid use of GOPRELTO in patients with a recent or active history of uncontrolled hypertension, unstable angina, myocardial infarction, coronary artery disease, or congestive heart failure. ( 5.3)ADVERSE REACTIONS
The most common adverse reactions (>0.5%) occurring in patients treated with GOPRELTO (cocaine hydrochloride) nasal solution 4% were headache and epistaxis. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact LXO US., INC. at 1-844-800-8007 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
• Disulfiram: Increases plasma cocaine exposure. Avoid using GOPRELTO in patients taking disulfiram. ( 7)
• Epinephrine, Phenylephrine: There have been reports of myocardial ischemia, myocardial infarction, and ventricular arrhythmias with concomitant use during nasal surgery. Avoid use of additional vasoconstrictor agents with GOPRELTO. If concomitant use is unavoidable, prolonged vital sign and ECG monitoring may be required. ( 5.3, 7)USE IN SPECIFIC POPULATIONS
• Pregnancy: May cause fetal harm. ( 8.1)
• Lactation: Avoid breastfeeding for 48 hours after treatment. ( 8.2)
• Hepatic Impairment: Monitor for adverse reactions such as headache, epistaxis, and clinically- relevant increases in heart rate or blood pressure. Do not administer a second dose within 24 hours of the first dose. ( 8.7)Revised: 11/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ABUSE AND DEPENDENCE
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Dosing Recommendation for Adults
2.3 Preparation and Administration of GOPRELTO Pledgets
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Abuse and Dependence
5.2 Seizures
5.3 Blood Pressure and Heart Rate Increases
5.4 Toxicology Screening
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
7 DRUG INTERACTIONS
7.1 Disulfiram
7.2 Epinephrine, Phenylephrine
7.3 Inhibitors of plasma cholinesterase (pseudocholinesterase)
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
8.8 Patients with Reduced Plasma Cholinesterase Activity
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ABUSE AND DEPENDENCE
CNS stimulants, including cocaine hydrochloride, have a high potential for abuse and dependence [see Warning and Precautions (5.1). ]
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
• GOPRELTO is for intranasal use only.
• Do not apply GOPRELTO to damaged nasal mucosa.2.2 Dosing Recommendation for Adults
The recommended dose of GOPRELTO is two soaked cottonoid pledgets placed in each nasal cavity, equivalent to 40 mg cocaine hydrochloride per pledget, for a total dose of 160 mg for four pledgets.
The total dose for any one procedure or surgery should not exceed 160 mg, or 3 mg/kg, cocaine hydrochloride. The recommended size of cottonoid pledgets for use with GOPRELTO measure 1.3 cm x 4 cm (sold separately).
2.3 Preparation and Administration of GOPRELTO Pledgets
Pour the full contents of one 4 mL (160 mg) bottle of GOPRELTO into a small container. Soak four cottonoid pledgets until the solution is fully absorbed.
Following soaking, place two pledgets in each nasal cavity against the septum.
Leave pledgets in place for up to twenty minutes. Remove pledgets and continue with the procedure.Discard pledgets and dispose of any unused portion of solution in accordance with institutional procedures for CII products. - 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Abuse and Dependence
Central nervous system (CNS) stimulants, including cocaine hydrochloride, have a high potential for abuse and dependence [ see Drug Abuse and Dependence ( 9.2, 9.3)].
5.2 Seizures
It has been reported in the literature that cocaine hydrochloride may lower the convulsive threshold. The risk may be higher in patients with a history of seizures or in patients with prior electroencephalogram (EEG) abnormalities without seizures, but has been reported in patients with no prior history or EEG evidence of seizures. Monitor patients for development of seizures.
5.3 Blood Pressure and Heart Rate Increases
As reported in the literature, cocaine hydrochloride causes an increase in observed blood pressure and heart rate. In the Phase 3 clinical study with GOPRELTO, increases in blood pressure and heart rate were observed for 60 minutes or longer following pledget removal. Monitor for vital sign changes, including heart rate and rhythm, after administration of GOPRELTO.
Avoid use of GOPRELTO in patients with a recent or active history of uncontrolled hypertension, unstable angina, myocardial infarction, coronary artery disease, or congestive heart failure. Avoid use of additional vasoconstrictor agents such as epinephrine or phenylephrine with GOPRELTO. If concomitant use is unavoidable, prolonged vital sign and ECG monitoring may be required [see Drug Interactions ( 7)].
-
6 ADVERSE REACTIONS
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
GOPRELTO has been evaluated in four Phase 1 studies and one Phase 3 study, which included 647 adult subjects who received a single topical intranasal 160 mg dose (four pledgets), of GOPRELTO. The randomized, double-blind, controlled Phase 3 study was conducted in adult patients undergoing diagnostic procedures and surgeries on or through the mucous membranes of the nasal cavities, of which 278 received GOPRELTO (4% solution), 275 received cocaine hydrochloride solution 8%, and 95 received placebo. Safety was evaluated for up to 7 days after dosing.
The most commonly reported adverse reactions (>1 patient) to occur in the Phase 3 study with GOPRELTO (4% solution) were headache and epistaxis. Two adverse reactions of headache were severe (Table 1).
No premature discontinuations due to an adverse event, serious adverse events, or deaths were reported in the Phase 3 clinical study.
Table 1: Common Adverse Reactions with GOPRELTO in >1 Patient System Organ Class / Preferred Term GOPRELTO 4%
(N=278)Cocaine Hydrochloride Solution 8%
(N=275)Placebo
(N=95)Nervous System Disorders Headache 7 (3%) 4 (2%) 1 (1%) Respiratory, Thoracic, and Mediastinal Disorders Epistaxis 3 (1%) 2 (1%) 0 Psychiatric Disorders Anxiety 0 2 (1%) 0 -
7 DRUG INTERACTIONS
7.1 Disulfiram
Published literature reported that disulfiram treatment increased plasma cocaine exposure, including both AUC and C max, by several fold after acute intranasal cocaine administration. Other literature reported that co-administration of disulfiram increased AUC of plasma cocaine by several fold after intravenous cocaine administration [see Clinical Pharmacology ( 12.3)]. Avoid using GOPRELTO in patients taking disulfiram. Consider using other local anesthetic agents.7.2 Epinephrine, Phenylephrine
There are reports in the published literature of myocardial ischemia, myocardial infarction, and ventricular arrhythmias after concomitant administration of topical intranasal cocaine with epinephrine and phenylephrine during nasal and sinus surgery.
Avoid use of additional vasoconstrictor agents such as epinephrine and phenylephrine with GOPRELTO during nasal and sinus surgery. If concomitant use is unavoidable, prolonged vital sign and ECG monitoring may be required [see Warnings and Precautions ( 5.3) ].7.3 Inhibitors of plasma cholinesterase (pseudocholinesterase)
Cocaine has been described in literature to be primarily metabolized and inactivated by nonenzymatic ester hydrolysis and hepatic carboxylesterase, and also by plasma cholinesterase, hepatic carboxylesterase, and CYP3A4 [see Clinical Pharmacology ( 12.3)]. The pharmacokinetics of GOPRELTO in patients with reduced plasma cholinesterase activity has not been studied.
Plasma cholinesterase activity may be decreased by chronic administration of certain monoamine oxidase inhibitors, oral contraceptives, or glucocorticoids. It may also be diminished by administration of irreversible plasma cholinesterase inhibitors such as echothiophate, organophosphate insecticides, and certain antineoplastic agents. Patients with reduced plasma cholinesterase (pseudocholinesterase) activity may have reduced clearance and increased exposure of plasma cocaine after administration of GOPRELTO.
Since cocaine is metabolized by multiple enzymes, the effect of reduced plasma cholinesterase activity on cocaine exposure may be limited. No dosage adjustment of GOPRELTO is needed in patients with reduced plasma cholinesterase. Monitor patients with reduced plasma cholinesterase activity for adverse reactions such as headache, epistaxis, and clinically-relevant increases in heart rate or blood pressure. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
In animal studies conducted in accordance with good laboratory practices, malformations including vertebral and rib abnormalities were reported when pregnant rabbits were treated with 16 mg/kg cocaine hydrochloride during organogenesis (8 times the adult human exposure following administration of pledgets containing 160 mg of cocaine) and increased pup mortality was noted when pregnant rats were exposed to cocaine hydrochloride during pregnancy at 47 times the adult human AUC exposures. Published rodent studies testing high exposures to cocaine during organogenesis report various malformations at 17 to 34 times the adult human AUC exposures following administration of pledgets containing 160 mg of cocaine.
Data
Human DataThere are no available data on the use of intranasal cocaine hydrochloride solution in pregnant women to inform a drug-associated risk adverse developmental outcomes. There are published data describing adverse developmental outcomes in women with chronic cocaine abuse during pregnancy. The published case-control and observational studies examining the effect of in utero cocaine exposure on fetal growth parameters, after controlling for confounding variables, found exposure was associated with reduced fetal growth compared with non-drugabuse populations. Published data from a large number of studies of women with chronic cocaine abuse during pregnancy are inconsistent in their findings with regard to other developmental outcomes.
Prospective studies controlling for polydrug use (marijuana, alcohol, tobacco) and lifestyle factors, have not demonstrated any association between cocaine abuse and specific major or minor fetal anomalies or other forms of fetal harm (premature birth, stillbirth, miscarriage, low birth weight, reduced head circumference, or placental abruption).The applicability of the findings from these studies of chronic abuse in pregnancy to a single topical exposure is limited.
Animal Data
No clear evidence of fetal malformations was noted in a study where pregnant rats were treated subcutaneously with up to 30 mg/kg cocaine hydrochloride (48 times the human adult exposure based on AUC following administration of pledgets containing 160 mg of cocaine) from Gestation Day 7 through 17 in the absence of overt maternal rat toxicity. However, a single high-dose fetus was reported with both meningoencephalocele and anophthalmia (unilateral).
Malformations, including vertebral and rib anomalies, were observed when pregnant rabbits were treated subcutaneously with 16 mg/kg cocaine hydrochloride (8 times the human adult exposure based on AUC exposures following administration of pledgets containing 160 mg of cocaine) from Gestation Day 7 through 20. This dose level was associated with evidence of maternal toxicity (convulsions, decreased body weight gain). No adverse effects were noted in animals treated with 8 mg/kg (1.5 times the adult human AUC following administration of pledgets containing 160 mg of cocaine).
An increased incidence of pup mortality between Postnatal Day (PND) 0 to PND 4 and decreased pup body weights from PND 1 to PND 21 were noted when pregnant rats were treated from GD 6 through Lactation Day 20 with 20 mg/kg cocaine hydrochloride (47 times the adult human AUC following administration of pledgets containing 160 mg of cocaine) in the absence of overt maternal toxicity. There was no adverse effect on pre- or postnatal development at 6 mg/kg (7 times the human adult AUC exposure following administration of pledgets containing 160 mg cocaine).Published studies in pregnant mice suggest that high exposure to cocaine (approximately 17-32 times the adult human AUC following administration of pledgets containing 160 mg of cocaine) produced adverse fetal effects including: exencephaly, cerebral hemorrhage,hydrocephalus, immaturely developed cerebral ventricles, limb anomalies, incomplete bone ossification, hydronephrosis, cryptorchidism, dilated or cystic ureters, and cleft lip/palate.
In a published nonhuman primate study, no adverse effects on physical development or cognitive function were noted after 1 mg/kg cocaine was administered via intramuscular injection three times a day (TID) (approximately 5 times the adult human AUC following administration of pledgets containing 160 mg of cocaine). However, higher exposures to cocaine decreased body weights, overall body length and crown circumference of offspring from pregnant Rhesus monkeys treated with escalating doses up to 7.5 mg/kg cocaine TID intramuscularity per day for 5 days per week from prior to conception to term (39 times the adult human AUC following administration of pledgets containing 160 mg of cocaine).
8.2 Lactation
Risk Summary
Based on limited case reports in published literature, cocaine is present in human milk at widely varying concentrations. Based on its pharmacochemical characteristics, high concentrations of cocaine are expected in breast milk with systemic exposure. The applicability of these findings to a single topical exposure with limited systemic absorption is unclear. No studies have evaluated cocaine concentrations in milk after topical administration of GOPRELTO.
Cocaine is detected in human breastmilk in chronic abuse situations and is expected to be at higher concentrations in milk than in maternal blood based on its physicochemical characteristics. Breastfeeding immediately after administration of GOPRELTO could result in infant plasma concentrations that are approximately half the anticipated maximum maternal plasma concentrations at the clinical dose of 160 mg. The effects of this cocaine plasma concentration in an infant are unknown, but no level of cocaine exposure is considered safe for a breastfed infant.
Adverse reactions have occurred in infants ingesting cocaine through breastmilk, including vomiting, diarrhea, convulsions, hypertension, tachycardia, agitation and irritability. The longterm effects on infants exposed to cocaine through breast milk are unknown. There are no data on the effects of GOPRELTO on milk production.
Because of the potential for serious adverse reactions in breastfed infants, advise nursing women that breastfeeding is not recommended during treatment with GOPRELTO and to pump and discard breastmilk for 48 hours after use of GOPRELTO.8.4 Pediatric Use
The safety and effectiveness of GOPRELTO in pediatric patients (17 years of age and younger) has not been evaluated.
Animal Data
Adverse CNS-related clinical signs within the first several days of dosing and decreased body weights were observed when juvenile rat pups were dosed subcutaneously with 25 mg/kg cocaine hydrochloride (15 and 32 times the adult human AUC exposure following administration of pledgets containing 160 mg of cocaine for males and females, respectively) from PND 7 to PND 28. No adverse effects were noted in pups dosed with 12.5 mg/kg (7 times the adult human AUC exposure following administration of pledgets containing 160 mg of cocaine).
A single mortality (male) and transient CNS signs were observed in male and female juvenile rat pups that were dosed subcutaneously up to 25 mg/kg cocaine hydrochloride (113 times the adult human AUC exposure) from PND 28 to PND 56. No adverse effects were noted in male pups dosed with 12.5 mg/kg or female pups dosed with 25 mg/kg (84 and 117 times the adult human AUC exposure following administration of pledgets containing 160 mg of cocaine respectively).8.5 Geriatric Use
Of the total number of subjects in the Phase 3 study, 12.1% of those who received GOPRELTO were 65 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience and pharmacokinetic data [see Clinical Pharmacology ( 12.3)] has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Renal Impairment
No dosage adjustment of GOPRELTO is needed in patients with mild, moderate, or severe renal impairment [ see Clinical Pharmacology ( 12.3)].
8.7 Hepatic Impairment
No dosage adjustment of GOPRELTO is needed in patients with hepatic impairment. Monitor patients with hepatic impairment for adverse reactions such as headache, epistaxis, and clinically- relevant increases in heart rate or blood pressure and do not administer a second dose of GOPRELTO to these patients within 24 hours of the first dose [ see Clinical Pharmacology ( 12.3)].
8.8 Patients with Reduced Plasma Cholinesterase Activity
Cocaine has been described in literature to be primarily metabolized and inactivated by nonenzymatic ester hydrolysis and hepatic carboxylesterase, and also by plasma cholinesterase, hepatic carboxylesterase and CYP3A4 [see Clinical Pharmacology ( 12.3)]. Pharmacokinetics of GOPRELTO in patients with reduced plasma cholinesterase activity has not been studied.
Genetic abnormalities of plasma cholinesterase (e.g., patients who are heterozygous or homozygous for atypical plasma cholinesterase gene), disease conditions such as malignant tumors, severe liver or kidney disease, decompensated heart disease, infections, burns, anemia, peptic ulcer, or myxedema or other physiological states such as pregnancy may lead to reduced plasma cholinesterase activity. Patients with reduced plasma cholinesterase (pseudocholinesterase) activity may have reduced clearance and increased exposure of plasma cocaine after administration of GOPRELTO.
Since cocaine is metabolized by multiple enzymes, the effect of reduced plasma cholinesterase activity on cocaine exposure may be limited. No dosage adjustment of GOPRELTO is needed in patients with reduced plasma cholinesterase. Monitor patients with reduced plasma cholinesterase activity for adverse reactions such as headache, epistaxis, and clinically-relevant increases in heart rate or blood pressure.
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
GOPRELTO contains cocaine, a substance with a high potential for abuse. GOPRELTO can be misused and abused, which can lead to addiction. GOPRELTO may also be diverted for abuse purposes [ see Warnings and Precautions ( 5.1)].
Drug abuse is the intentional non-therapeutic use of a prescription drug, even once, for its rewarding psychological or physiological effects. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that develop after repeated substance use and includes: a strong desire to take the drug, difficulties in controlling its use, persisting in its use despite harmful consequences, a higher priority given to drug use than to other activities and obligations, increased tolerance, and sometimes a physical withdrawal. Drug abuse of a substance may occur without progression to drug addiction. “Drug-seeking” behavior is very common in persons with substance use disorders.
Drug abuse and addiction are conditions that are separate and distinct from physical dependence and tolerance [see Dependence ( 9.3)]. Health care providers should be aware that abuse and addiction may occur in the absence of symptoms indicative of physical dependence and tolerance.
Individuals who abuse stimulants may use GOPRELTO for abuse purposes. Adverse events associated with abuse of cocaine include euphoria, excitation, irritability, restlessness, anxiety, paranoia, confusion, headache, psychosis, hypertension, stroke, seizures, dilated pupils, nausea, vomiting, and abdominal pain. Intranasal abuse can produce damage to the nostrils (e.g.,ulceration and deviated septum). Abuse of cocaine can result in overdose, convulsions, unconsciousness, coma, and death [ see Overdosage ( 10)]. Parenteral drug abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.GOPRELTO, like all prescription drugs with abuse potential, can be diverted for non-medical use into illicit channels of distribution. In order to minimize these risks, effective accounting procedures should be implemented, in addition to routine procedures for handling controlled substances.
9.3 Dependence
Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. GOPRELTO is approved for topical single use during diagnostic procedures and surgeries, so physical dependence and withdrawal symptoms are unlikely to develop. Although GOPRELTO is not indicated for chronic therapy, repeated misuse or abuse of this product may lead to physical dependence.
-
10 OVERDOSAGE
No cases of overdose with GOPRELTO were reported in clinical trials. Blood pressure and heart rate increases were greater with cocaine hydrochloride solution 8% than with GOPRELTO. In the case of an overdose, consult with a certified poison control center (1-800-222-1222) for up-to-date guidance and advice for treatment of overdosage. Individual patient response to cocaine varies widely. Toxic symptoms may occur idiosyncratically at low doses. Manifestations of cocaine overdose associated with illicit use of cocaine reported in literature and based on reports in FDA’s Adverse Events Reporting System (AERS) database include death, cardio-respiratory arrest, cardiac arrest, respiratory arrest, tachycardia, myocardial infarction, agitation, aggression, restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia, and rhabdomyolysis. Fatigue
and depression usually follow the central nervous system stimulation. Other reactions include arrhythmias, hypertension or hypotension, circulatory collapse, nausea, vomiting, diarrhea, and abdominal cramps. Fatal poisoning is usually preceded by convulsions and coma. Because cocaine is significantly distributed to tissues and rapidly metabolized, dialysis and hemoperfusion are not effective. Acidification of the urine does not significantly enhance cocaine elimination. -
11 DESCRIPTION
GOPRELTO (cocaine hydrochloride) nasal solution for intranasal use contains a 4% solution, 160 mg/4 mL (40 mg/mL), equivalent to 142.4 mg/4 mL (35.6 mg/mL) cocaine, an ester local anesthetic.
The chemical name for cocaine hydrochloride is (1R,2R,3S,5S) methyl 3-(benzoyloxy)-8- methyl-8-azabicyclo[3.2.1]octane-2-carboxylate hydrochloride. The molecular formula is C17H21NO4•HCl and the molecular weight is 339.81. The structural formula is: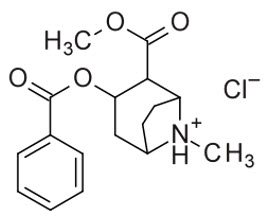
Inactive ingredients are anhydrous citric acid, D&C Yellow No. 10, FD&C Green No. 3, sodium benzoate, and purified water.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Cocaine hydrochloride is a local anesthetic of the ester type. Cocaine hydrochloride prevents conduction in nerve fibers by reversibly blocking sodium channels and preventing the transient rise in sodium conductance necessary for generation of an action potential.
12.2 Pharmacodynamics
Cardiac Electrophysiology
The effect of GOPRELTO (cocaine hydrochloride) nasal solution on the QTc interval was evaluated in a randomized, positive- and placebo-controlled four-period crossover thorough QTc study in 24 healthy subjects. No clinically relevant QTc prolongation was observed at the highest clinically relevant concentrations with a single therapeutic dose.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics of GOPRELTO (cocaine hydrochloride) nasal solution have been assessed in 74 healthy adult subjects across 4 studies. Following intranasal application of two 40 mg pledgets applied to each nasal cavity (160 mg cocaine hydrochloride total dose) for 20 minutes, the geometric mean (SD) cocaine C max was 43.2 (1.73) ng/mL. The median (range) time to peak plasma concentration (t max) was 0.42 (0.25 – 1.75) hours after pledget application.Distribution
Cocaine has been described in literature as approximately 84 – 92% bound to human plasma proteins, binding primarily to alpha-1-acid glycoprotein (AAG) and albumin.
In studies with GOPRELTO, the apparent volume of distribution (Vd/F) of cocaine after intranasal administration is 3,877 ± 1,266 L.Elimination
Metabolism
Cocaine has been described in literature to be primarily metabolized and inactivated by nonenzymatic ester hydrolysis and hepatic carboxylesterase 1 to form benzoylecgonine (BE), and by plasma cholinesterase and hepatic carboxylesterase 2 to form ecgonine methyl ester (EME). In human liver microsomes, cocaine undergoes CYP3A4 mediated N-demethylation to produce a minor metabolite, norcocaine, which is pharmacologically active.Excretion
Cocaine has been described in literature to be primarily eliminated by biotransformation to inactive metabolites, BE and EME. Less than 10% of the administered dose is excreted unchanged in the urine. BE and EME are both predominantly excreted by the kidneys.In studies with GOPRELTO, 0-32 hour urinary recoveries of cocaine, BE, and EME as a percentage of dose were approximately 0.1%, 2.0%, and 1.0%, respectively. The mean elimination half-life of cocaine was 1.0 to 1.7 hours; with longer plasma sampling (32 hours) and a highly sensitive assay, mean half-life values of 5.0 to 8.0 hours were observed at very low plasma concentrations.
The apparent clearance of cocaine after intranasal administration of GOPRELTO (CL/F) is 3096 ± 1276 L/h.
Specific Populations
In studies with GOPRELTO, cocaine exposure (i.e., C max, AUC last, and AUC inf) was slightly higher in females than males whereas tmax and half-life were similar in males and females. GOPRELTO pharmacokinetics are not affected by age or weight.
Renal Impairment
In a pharmacokinetic study of GOPRELTO in subjects with normal and severe renal impairment (eGFR 15-29 mL/min/1.73 m2), mean AUC and Cmax were slightly higher in subjects with severe renal impairment compared to those with normal renal function and clearance was slightly lower [see Use in Specific Populations ( 8.6)].
Hepatic Impairment
In a pharmacokinetic study of GOPRELTO in subjects with normal, Child-Pugh Class B, and Child-Pugh Grade C hepatic impairment, there was a minimal effect of hepatic impairment on cocaine C max. In moderately impaired subjects (n=9) there was a higher than two-fold increase in AUC (79.2 ng.h/mL in normal subjects to 225 ng.h/mL in Child-Pugh Grade B subjects) and the clearance was reduced by more than half (1735 L/h in normal 629 L/h in Child-Pugh Grade B subjects). In severely impaired subjects (n=3) there was an eighty percent increase in AUC (79.2 ng.h/mL in normal subjects to 142 ng.h/mL in Child-Pugh Grade C subjects) and the clearance was reduced to half (1735 L/h in normal 959 L/h in Child-Pugh Grade C subjects) [see Use in Specific Populations ( 8.7)].
Drug Interaction Studies
Cocaine has been found to be a CYP2D6 inhibitor in in-vitro studies employing human liver microsomes. In vitro transporter inhibition studies also found cocaine to be an inhibitor of OCT2. However, the relatively low plasma concentrations of cocaine resulting from therapeutic doses of GOPRELTO are not expected to raise significant drug-drug interaction concerns.Disulfiram
It has been reported in the published literature that disulfiram treatment increased plasma cocaine exposure, including both AUC and C max, by several fold after acute intranasal cocaine administration. Other published literature reported that co-administration of disulfiram increased AUC of plasma cocaine by several fold after intravenous cocaine administration [see Drug Interactions ( 7.1)]. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies to evaluate the carcinogenic potential of cocaine have not been conducted.Mutagenesis
In published studies, cocaine was genotoxic in the in vitro chromosomal aberration assay, the in vitro sister chromatid exchange assay, the in vitro micronucleus assay, and the in vitro hypoxanthine-guanine phosphoribosyltransferase (hgprt) assay. Cocaine was equivocal in a published in vivo micronucleus assay and the in vivo comet assay (liver). Cocaine was not mutagenic in the in vitro bacterial reverse mutation assay (Ames assay).Impairment of Fertility
No adverse effects on fertility or early embryonic development were reported in a study where male rats were administered up to 20 mg/kg cocaine hydrochloride via subcutaneous injection for 28 days prior to mating and female rats were treated with the same dose for 14 days prior to mating through Gestation Day 7. The 20 mg/kg/dose resulted in AUC exposures that were 31 times (males) and 47 times (females) the adult human AUC following administration of pledgets containing 160 mg of cocaine.
-
14 CLINICAL STUDIES
A double-blind, multicenter, single-dose, placebo- and dose-controlled, parallel-group study was conducted in 648 subjects undergoing diagnostic procedures and surgeries on or through the mucous membranes of the nasal cavities. Subjects were randomized to receive GOPRELTO (n=278), cocaine hydrochloride solution 8% (n=275), to explore the dosing range, or placebo (n=95). Nasal endoscopy, nasal laryngoscopy, nasopharyngeal laryngoscopy, and nasal debridement comprised 88% of all procedures performed in the GOPRELTO group and 85% of all procedures performed in the placebo group. All subjects completed the diagnostic or surgical procedure.
In the GOPRELTO group, two 40 mg pledgets were applied to the septum in each nasal cavity (160 mg cocaine hydrochloride total dose) and left in place for up to 20 minutes. Similarly, pledgets were applied in the placebo group. Topical anesthesia was assessed using the visual numeric rating scale (VNRS) during a von Frey Filament test prior to the diagnostic procedure or surgery. After subject-reported pain scores were collected, the blind to placebo was broken and placebo subjects were provided the option of receiving anesthesia. The primary efficacy endpoint was analgesic success, defined in the GOPRELTO group as a subject-reported pain score of 0 (no pain) on the VNRS during the von Frey Filament test, and no additional anesthetic or analgesic medication administration during the diagnostic procedure or surgery. Analgesic success was defined in the placebo group as a subject-reported pain score of 0 on the VNRS during the von Frey Filament test. Subjects did not receive supplemental intravenous sedation or general anesthesia during the study.
Table 2 provides the efficacy results for the primary endpoint of analgesic success showing a significant difference in the analgesic success rate between placebo and GOPRELTO.
Table 2: Analgesic Success Event GOPRELTO
(N=278)
n (%)Placebo
(N=95)
n (%)Success 215 (77%) 14 (15%) Failure 63 (23%) 81 (85%) Of the 63 (23%) failures in the GOPRELTO group, 4 subjects requested additional anesthetic medication. Of these 4 subjects, 1 subject reported 0 on the VNRS during the von Frey Filament test. Of the 81 (85%) failures in the placebo group, 50 subjects required additional anesthetic medication.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
GOPRELTO (cocaine hydrochloride) nasal solution is a clear, green colored liquid available as one dosage strength:
160 mg/4 mL (40 mg/mL or 4%) cocaine hydrochloride, equivalent to 142.4 mg/4 mL (35.6 mg/mL) cocaine
NDC # 70839-359-04: Single-unit 4 mL bottle
Store upright at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F)
[ see USP, Controlled Room Temperature (CRT)]. Avoid freezing. -
17 PATIENT COUNSELING INFORMATION
Potential for Abuse and Dependence
Advise patients that GOPRELTO is a controlled substance and it can be abused and lead to dependence [see Warnings and Precautions( 5.1), Drug Abuse and Dependence ( 9)].
Toxicology Screening
Advise patients that the cocaine hydrochloride in GOPRELTO may be detected in plasma for up to one week after administration. Cocaine hydrochloride and its metabolites may be detected in urine toxicology screening for longer than one week after administration. [ see Warnings and Precautions ( 5.4)].
Seizures
Advise patients that GOPRELTO may lower the seizure threshold. Patients should be monitored for development of seizures. [ see Warnings and Precautions ( 5.2) ].
Blood Pressure and Heart Rate Increase
Advise patients that GOPRELTO can cause increases in blood pressure and heart rate and should be avoided in patients with recent or active history of uncontrolled hypertension, unstable angina, myocardial infarction, coronary artery disease, or congestive heart failure [ see Warnings and Precautions ( 5.3) ].Headache and/or Epistaxis
Inform patients that headache and/or epistaxis are the most frequently experienced side effects that should resolve without treatment. Instruct patients to contact their health care professional if these symptoms persist [ see Adverse Reactions ( 6) ].
Pregnancy
Inform female patients of reproductive potential that GOPRELTO may cause fetal harm and to inform their prescriber of a known or suspected pregnancy [ see Use in Specific Populations ( 8.1)] .
Lactation
Advise a nursing woman that breastfeeding is not recommended during treatment with GOPRELTO and to pump and discard breastmilk for 48 hours after administration of GOPRELTO nasal solution [ see Use in Specific Populations ( 8.2) ]Manufactured by:
Genus Lifesciences Inc.
514 North 12th Street
Allentown, PA 18102Distributed by:
LXO US, INC.
1690 Sumneytown Pike
Suite 250
Lansdale, PA 19446 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
GOPRELTO
cocaine hydrochloride solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70839-359 Route of Administration NASAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength COCAINE HYDROCHLORIDE (UNII: XH8T8T6WZH) (COCAINE - UNII:I5Y540LHVR) COCAINE HYDROCHLORIDE 40 mg in 1 mL Inactive Ingredients Ingredient Name Strength FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) Product Characteristics Color green Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70839-359-04 1 in 1 CARTON 02/09/2024 1 4 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA209963 02/09/2024 Labeler - LXO US Inc. (932134773) Establishment Name Address ID/FEI Business Operations Genus Lifesciences Inc. 113290444 manufacture(70839-359)