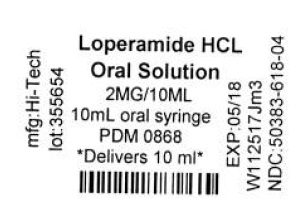
Label: LOPERAMIDE HYDROCHLORIDE solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 68151-0618-1 - Packager: Carilion Materials Management
- This is a repackaged label.
- Source NDC Code(s): 50383-618
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 28, 2017
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
Alergy alert: Do not use if you have ever had a rash or other allergic reaction to loperamide HCl
Do not use if you have bloody or black stool
Ask a doctor before use if you have
- high fever (greater than 101°F)
- mucus present in your stool
- a history of liver disease
Ask doctor or pharmacist before use if you are taking antibiotics
Stop use and ask a doctor if diarrhea lasts for more than 2 days
If pregnant or breast-feeding, ask a health professional before use
-
Directions
- use enclosed dosage cup to accurately measure dosage as noted below
- drink plenty of clear fluids to help prevent dehydration, which may accompany diarrhea
- find right dose on chart. If possible, use weight to dose; otherwise, use age.
adults and children 12 years and older 4 teaspoonfuls (1 dosage cup) after the first loose bowel movement; 2 teaspoonfuls (1/2 dosage cup) after each subsequent loose bowel movement; but no more than 8 teaspoonfuls a day children 9-11 years (60-95 lbs) 2 teaspoonfuls (1/2 dosage cup) after the first loose bowel movement; 1 teaspoonful (1/4 dosage cup) after each subsequent loose bowel movement; but no more than 6 teaspoonfuls a day children 6-8 years (48-59 lbs) 2 teaspoonfuls (1/2 dosage cup) after the first loose bowel movement; 1 teaspoonful (1/4 dosage cup) after each subsequent loose bowel movement; but no more than 4 teaspoonfuls a day children under 6 years (up to 47 lbs) ask a doctor (not intended for use in children under 6 years old) - Other information
- Inactive ingredients
- Questions or comments?
- HOW SUPPLIED
- LOPERAMIDE HYDROCHLORIDE SOLUTION
-
INGREDIENTS AND APPEARANCE
LOPERAMIDE HYDROCHLORIDE
loperamide hydrochloride solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68151-0618(NDC:50383-618) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LOPERAMIDE HYDROCHLORIDE (UNII: 77TI35393C) (LOPERAMIDE - UNII:6X9OC3H4II) LOPERAMIDE HYDROCHLORIDE 1 mg in 5 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BENZOIC ACID (UNII: 8SKN0B0MIM) CHERRY (UNII: BUC5I9595W) GLYCERIN (UNII: PDC6A3C0OX) MINT (UNII: FV98Z8GITP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68151-0618-1 1 mL in 1 SYRINGE, PLASTIC; Type 0: Not a Combination Product 12/28/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074352 11/17/1995 Labeler - Carilion Materials Management (079239644) Establishment Name Address ID/FEI Business Operations Carilion Materials Management 079239644 REPACK(68151-0618)