Label: LINCOMYCIN injection
- NDC Code(s): 46066-502-02
- Packager: Aspen Veterinary Resources
- Category: PRESCRIPTION ANIMAL DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Animal Drug Application
Drug Label Information
Updated March 13, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION
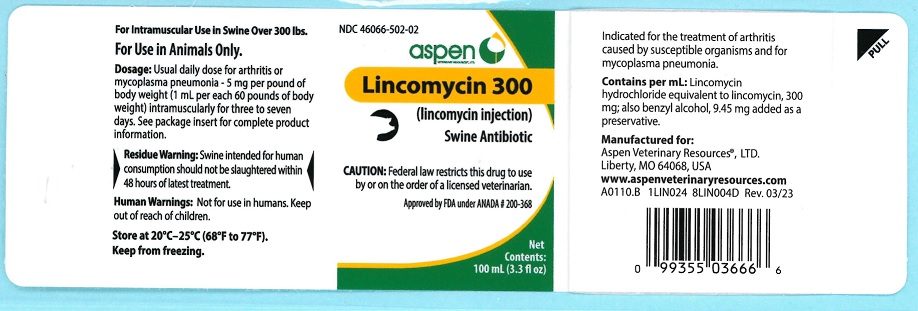
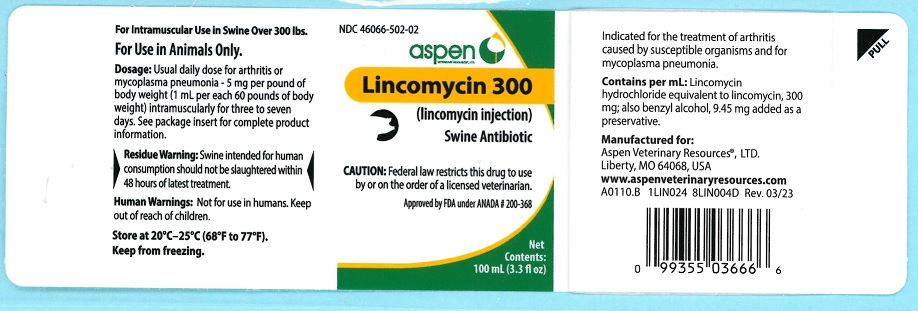
Lincomycin 300
(lincomycin injection)For use in swine weighing 300 pounds or more.
For Intramuscular Use in Swine OnlyCAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
Lincomycin 300 contains lincomycin hydrochloride, an antibiotic produced by Streptomyces lincolnensis var. lincolnensis which is chemically distinct from all other clinically available antibiotics and is isolated as a white crystalline solid.
-
INDICATIONS & USAGE
INDICATIONS FOR SWINE:
Lincomycin 300 is indicated for the treatment of infectious forms of arthritis caused by organisms sensitive to its activity. This includes most of the organisms responsible for the various infectious arthritides in swine, such as staphylococci, streptococci, Erysipelothrix and Mycoplasma spp.
It is also indicated for the treatment of mycoplasma pneumonia. - CONTRAINDICATIONS
- RESIDUE WARNING
- USER SAFETY WARNINGS
- GENERAL PRECAUTIONS
-
ADVERSE REACTIONS
ADVERSE REACTIONS:
The intramuscular administration to swine may cause a transient diarrhea or loose stools. Although this effect has rarely been reported, one must be alert to the possibility that it may occur. Should this occur, it is important that the necessary steps be taken to prevent the effects of dehydration.The Safety Data Sheet (SDS) contains more detailed occupational safety information. To report adverse drug events, for technical assistance or to obtain a copy of the SDS, contact Aspen Veterinary Resources at info@aspenveterinaryresources.com. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or online at www.fda.gov/reportanimalae.
-
DOSAGE & ADMINISTRATION
DOSAGE AND ADMINISTRATION:
For arthritis or mycoplasma pneumonia - 5 mg per pound of body weight intramuscularly once daily for three to seven days as needed. One mL per 60 lb body weight will provide 5 mg/lb.For optimal results, initiate treatment as soon as possible.
As with any multi-dose vial, practice aseptic techniques in withdrawing each dose. Adequately clean and disinfect the vial closure prior to entry with a sterile needle and syringe. No vial closure should be entered more than 20 times.
Each mL contains:
Lincomycin hydrochloride equivalent to lincomycin 300mg; also benzyl alcohol 9.45 mg added as a preservative. - HOW SUPPLIED
- STORAGE AND HANDLING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LINCOMYCIN
lincomycin injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC:46066-502 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LINCOMYCIN HYDROCHLORIDE (UNII: M6T05Z2B68) (LINCOMYCIN - UNII:BOD072YW0F) LINCOMYCIN 300 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:46066-502-02 100 mL in 1 VIAL, MULTI-DOSE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANADA ANADA200368 06/30/2008 Labeler - Aspen Veterinary Resources (627265361) Registrant - Bimeda, Inc. (060492923) Establishment Name Address ID/FEI Business Operations Bimeda-MTC 256232216 manufacture