Label: DR MANALI HAIR SERUM FOR MEN- minoxidil 5% liquid
- NDC Code(s): 83361-002-01, 83361-002-02
- Packager: Dr Manali Products
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
-
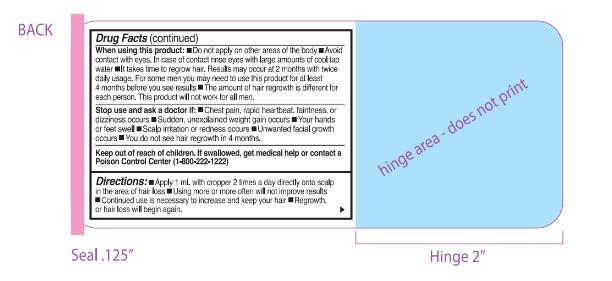
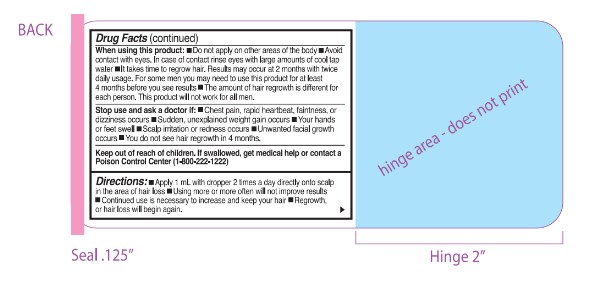
WARNINGS
Warnings: For eldemal use only. For use by men only. Flarnmabk!: Keep away from fire or flame
Warnings: Do not use 11:■You are a woman ■ You have no family histcxy of hair loss ■ Your hair loss is sudden or patchy ■ You do not know the reason for your hair loss ■You do not know the reason for your hair loss you are under 18 years of age.■ Do not use on babies or children ■Your scalp is red, inflamed, infected, irritated, or painful ■ You use other medications on the scalp
Ask a doctor before use if you have heart disease.
-
WHEN USING
When using this product: ■ Do not apply on other areas of the body ■ Avoid contact with eyes. In case of contact rinse eyes wnh large announts of cool tap water ■ It takes time to regrow hair. Results may occur at 2 months h twice daily usage. For some men you may need to use this product for at least
4 months before you see results ■The annount of hair regrowth is different for each person. This product will not work for all men.
-
DO NOT USE
Do not use if :■You are a woman ■ You have no family history of hair loss ■ Your hair loss is sudden or patchy ■ You do not know the reason for your hair loss ■You do not know the reason for your hair loss you are under 18 years of age.■ Do not use on babies or children ■Your scalp is red, inflamed, infected, irritated, or painful ■ You use other medications on the scalp
- ASK DOCTOR
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- STORAGE AND HANDLING
-
INACTIVE INGREDIENT
Inactive Ingredients: Biotin Liposomes (water, alcohol, panthenol, lecithin, tocopheryl acetate, caffeine, biotin), Ethyl Alcohol, Dimethyl lsosorbide, Melatonin, Prickly Pear Extract (glycerin & water & Opuntia Tuna Flower/Stem Extract), Propylene Glycol, Red Clover Extract (Certified Organic Red Clover (Trifolium Pratense) dried Leal, vegetable glycerin, alcohol, water], Saw Palmetto/Serenoa repens Extract (glycerin, water, Serenoa Serrulata Fruit Extract, Maltodextrin), Vitamin A Liposomes (lecithin, retinal, polysorbate 20)
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DR MANALI HAIR SERUM FOR MEN
minoxidil 5% liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83361-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) MELATONIN (UNII: JL5DK93RCL) POLYSORBATE 20 (UNII: 7T1F30V5YH) ALCOHOL (UNII: 3K9958V90M) PANTHENOL (UNII: WV9CM0O67Z) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) BIOTIN (UNII: 6SO6U10H04) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) VITAMIN A (UNII: 81G40H8B0T) PRICKLY PEAR FRUIT (UNII: 18V8PAQ629) RED CLOVER (UNII: L9153EKV2Y) TRIFOLIUM PRATENSE LEAF (UNII: 612YOZ36DG) WATER (UNII: 059QF0KO0R) MALTODEXTRIN (UNII: 7CVR7L4A2D) GLYCERIN (UNII: PDC6A3C0OX) SAW PALMETTO (UNII: J7WWH9M8QS) CAFFEINE (UNII: 3G6A5W338E) OPUNTIA TUNA STEM (UNII: 2HCC68I98K) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) RETINOL (UNII: G2SH0XKK91) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83361-002-02 1 in 1 CARTON 02/01/2024 1 NDC:83361-002-01 60 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M032 02/01/2024 Labeler - Dr Manali Products (110035406) Establishment Name Address ID/FEI Business Operations Dr Manali Products 110035406 manufacture(83361-002)