Label: BLISS REPUBLIC LIDOCAINE NUMBING CREAM- lidocaine numbing cream cream
- NDC Code(s): 84028-111-11, 84028-111-12, 84028-111-13
- Packager: Sargass Group Limited Liability Company
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated February 16, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
-
WHEN USING
When using this product:
Adhere to the recommended daily dosage unless otherwise directed by a doctor.
Some individuals with heightened sensitivity might experience allergic reactions to specific ingredients in this product.Do not insert this cream into the rectum using fingers, applicators, or any medical devices.
- KEEP OUT OF REACH OF CHILDREN
- STOP USE
- Directions
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
BLISS REPUBLIC LIDOCAINE NUMBING CREAM
lidocaine numbing cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84028-111 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 5 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) MENTHOL (UNII: L7T10EIP3A) CARBOMER 934 (UNII: Z135WT9208) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) BENZYL ALCOHOL (UNII: LKG8494WBH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84028-111-11 30 mL in 1 JAR; Type 0: Not a Combination Product 01/03/2024 2 NDC:84028-111-12 55 mL in 1 JAR; Type 0: Not a Combination Product 01/03/2024 3 NDC:84028-111-13 100 mL in 1 JAR; Type 0: Not a Combination Product 01/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/03/2024 Labeler - Sargass Group Limited Liability Company (119155798)

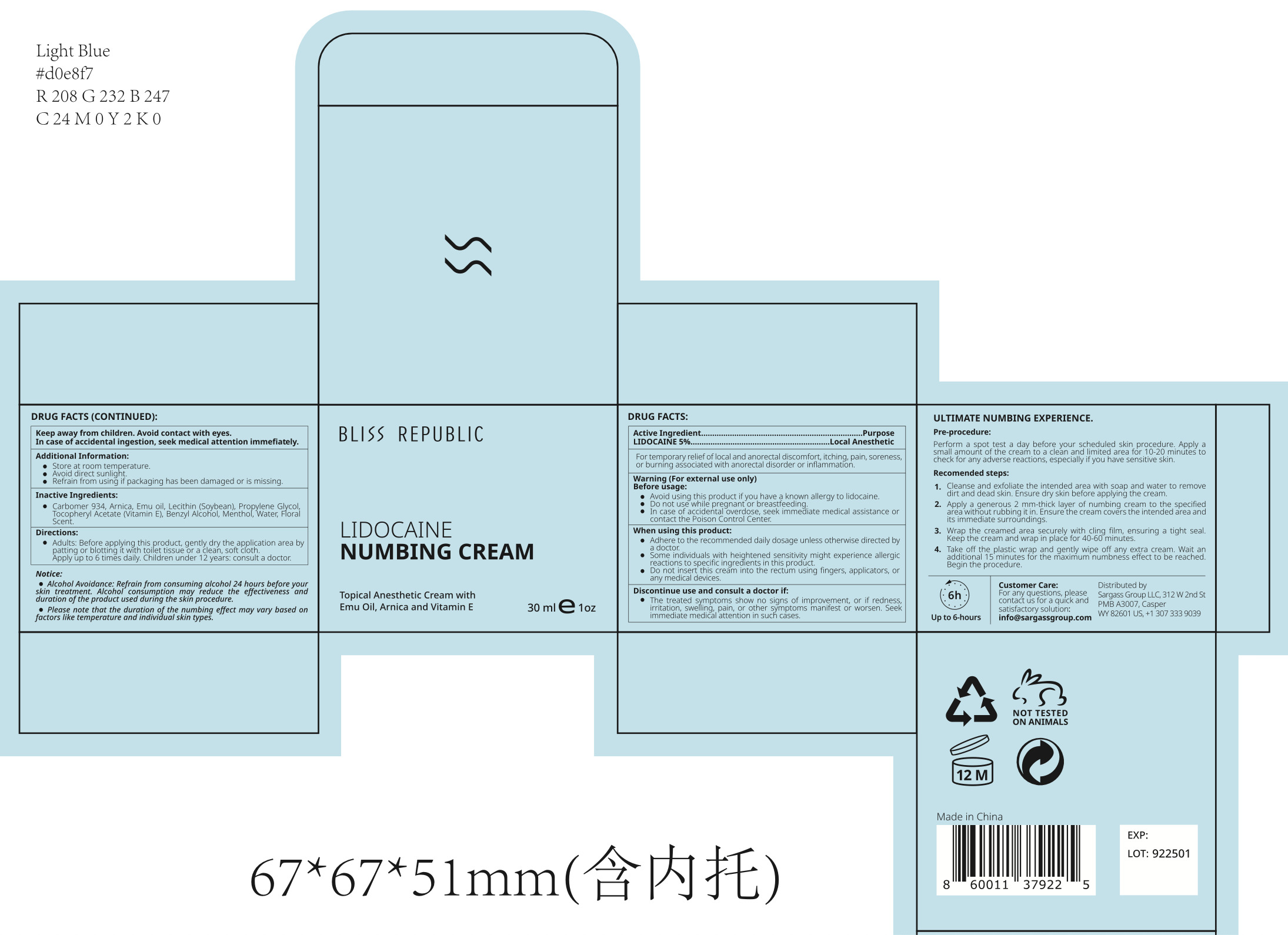
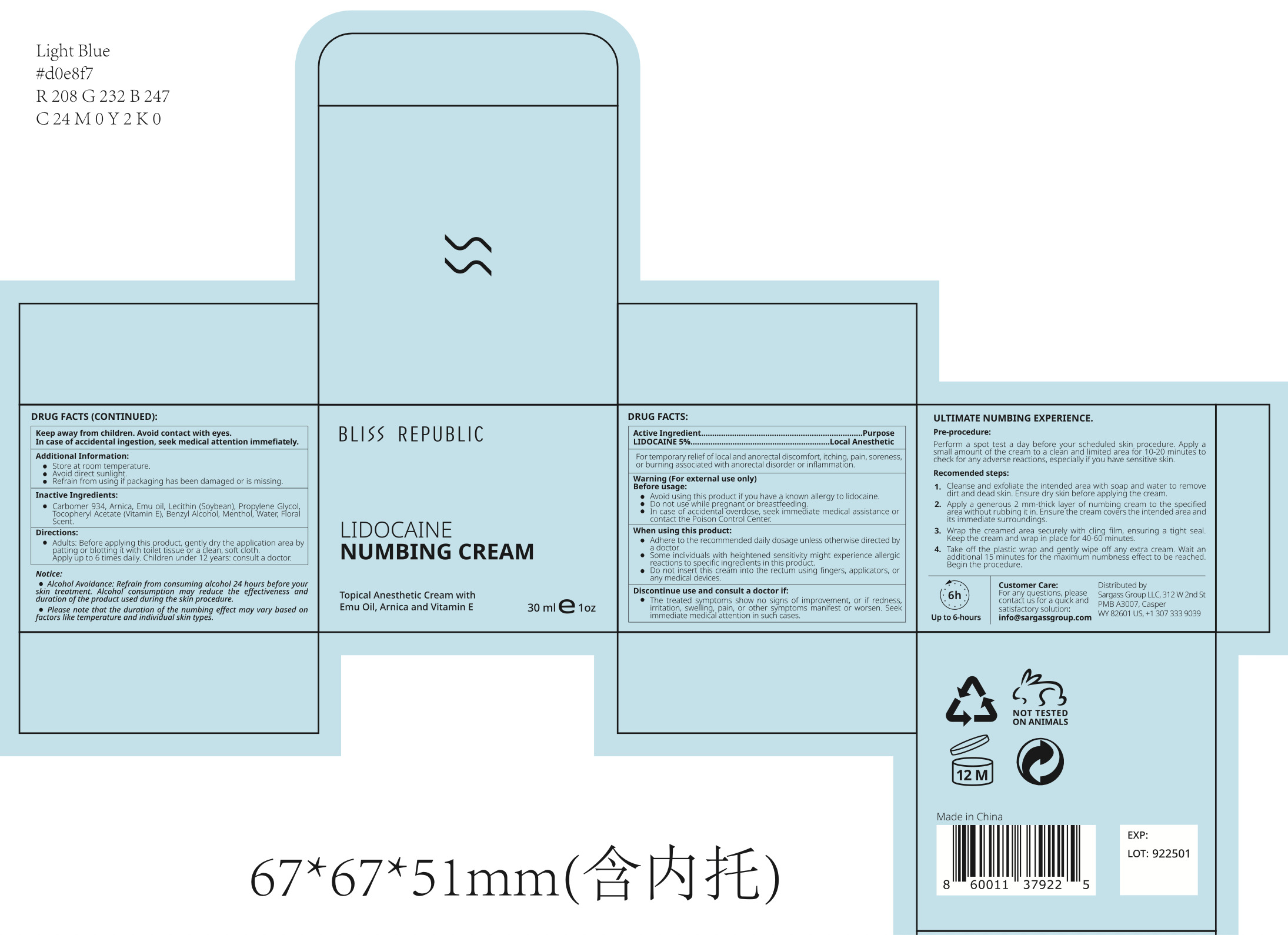 30mL NDC: 84028-111-11
30mL NDC: 84028-111-11