Label: THIAMINE HYDROCHLORIDE injection, solution
- NDC Code(s): 70518-4230-0, 70518-4230-1
- Packager: REMEDYREPACK INC.
- This is a repackaged label.
- Source NDC Code(s): 0641-6228
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 14, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
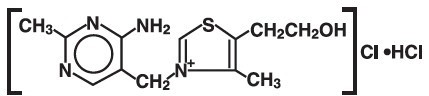
DESCRIPTIONThiamine Hydrochloride Injection, USP is a sterile solution of thiamine hydrochloride in Water for Injection for intramuscular (IM) or slow intravenous (IV) administration. Each mL contains ...
-
CLINICAL PHARMACOLOGYThe water soluble vitamins are widely distributed in both plants and animals. They are absorbed in man by both diffusion and active transport mechanisms. These vitamins are structurally diverse ...
-
INDICATIONSThiamine hydrochloride injection is effective for the treatment of thiamine deficiency or beriberi whether of the dry (major symptoms related to the nervous system) or wet (major symptoms related ...
-
CONTRAINDICATIONSA history of sensitivity to thiamine or to any of the ingredients in this drug is a contraindication. (See - WARNINGS - for further information.)
-
WARNINGSWARNING: This product contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are ...
-
PRECAUTIONSGeneral - Simple vitamin B - 1deficiency is rare. Multiple vitamin deficiencies should be suspected in any case of dietary inadequacy. Information for Patients - The patient should be ...
-
ADVERSE REACTIONSTo report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch. An occasional individual may develop a ...
-
OVERDOSAGEParenteral doses of 100 to 500 mg singly have been administered without toxic effects. However, dosages exceeding 30 mg three times a day are not utilized effectively. When the body tissues are ...
-
DOSAGE AND ADMINISTRATION“Wet” beriberi with myocardial failure must be treated as an emergency cardiac condition, and thiamine must be administered slowly by the IV route in this situation (see - WARNINGS). In the ...
-
HOW SUPPLIEDThiamine Hydrochloride Injection, USP is supplied as follows: 200 mg per 2 mL (100 mg per mL) multiple dose vials packed in cartons containing 25 vials per carton. NDC: 70518-4230-00 - NDC ...
-
PRINCIPAL DISPLAY PANELDRUG: Thiamine Hydrochloride - GENERIC: Thiamine Hydrochloride - DOSAGE: INJECTION, SOLUTION - ADMINSTRATION: INTRAMUSCULAR - NDC: 70518-4230-0 - NDC: 70518-4230-1 - PACKAGING: 1 in 1 VIAL - OUTER PACKAGING: 25 ...
-
INGREDIENTS AND APPEARANCEProduct Information