Label: REVITALIZING SUPREME YOUTH POWER CREME BROAD SPECTRUM SPF 25- avobenzone,homosalate,octisalate, and octocrylene cream
- NDC Code(s): 11559-065-01, 11559-065-02
- Packager: ESTEE LAUDER INC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 25, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
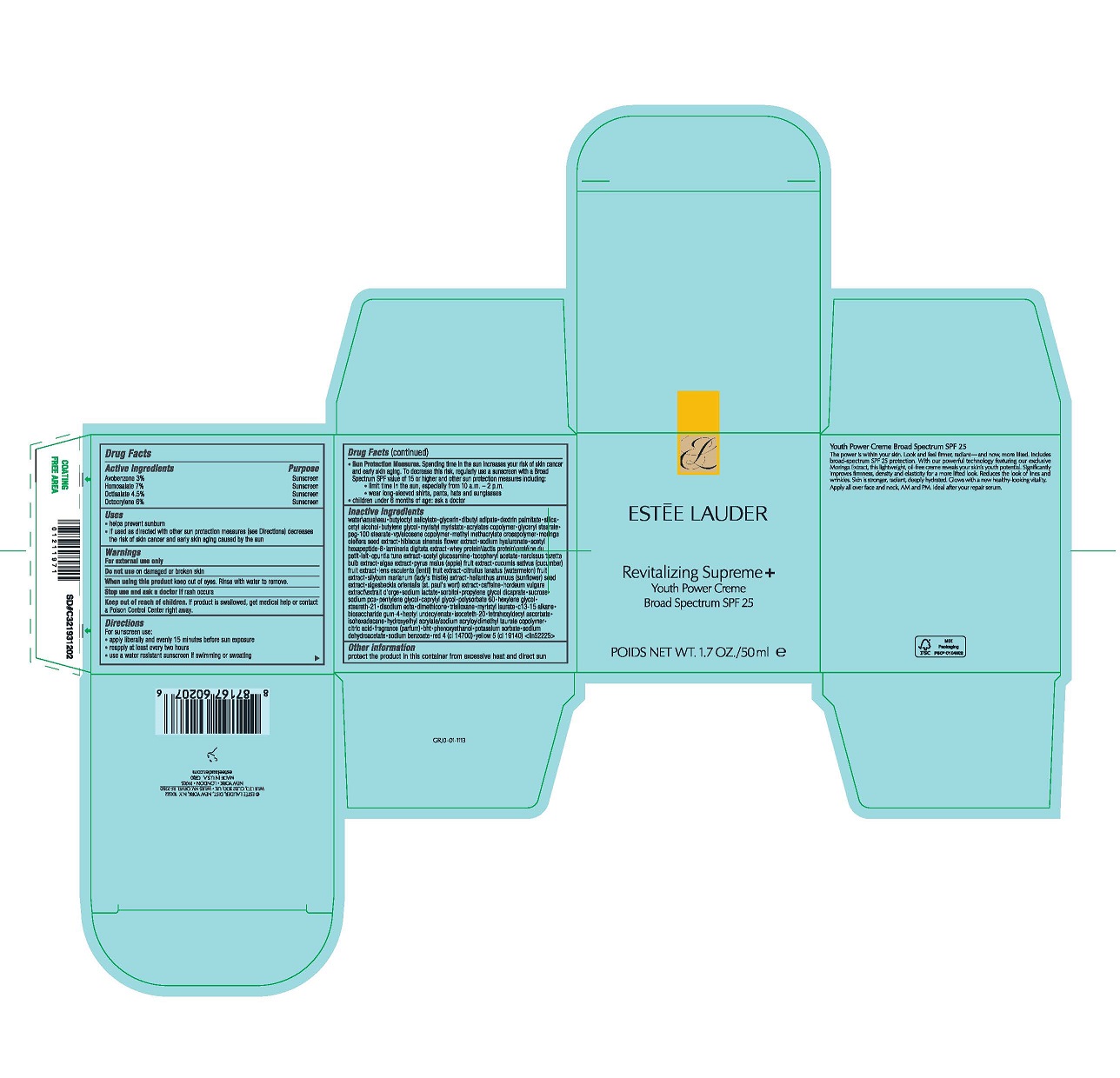
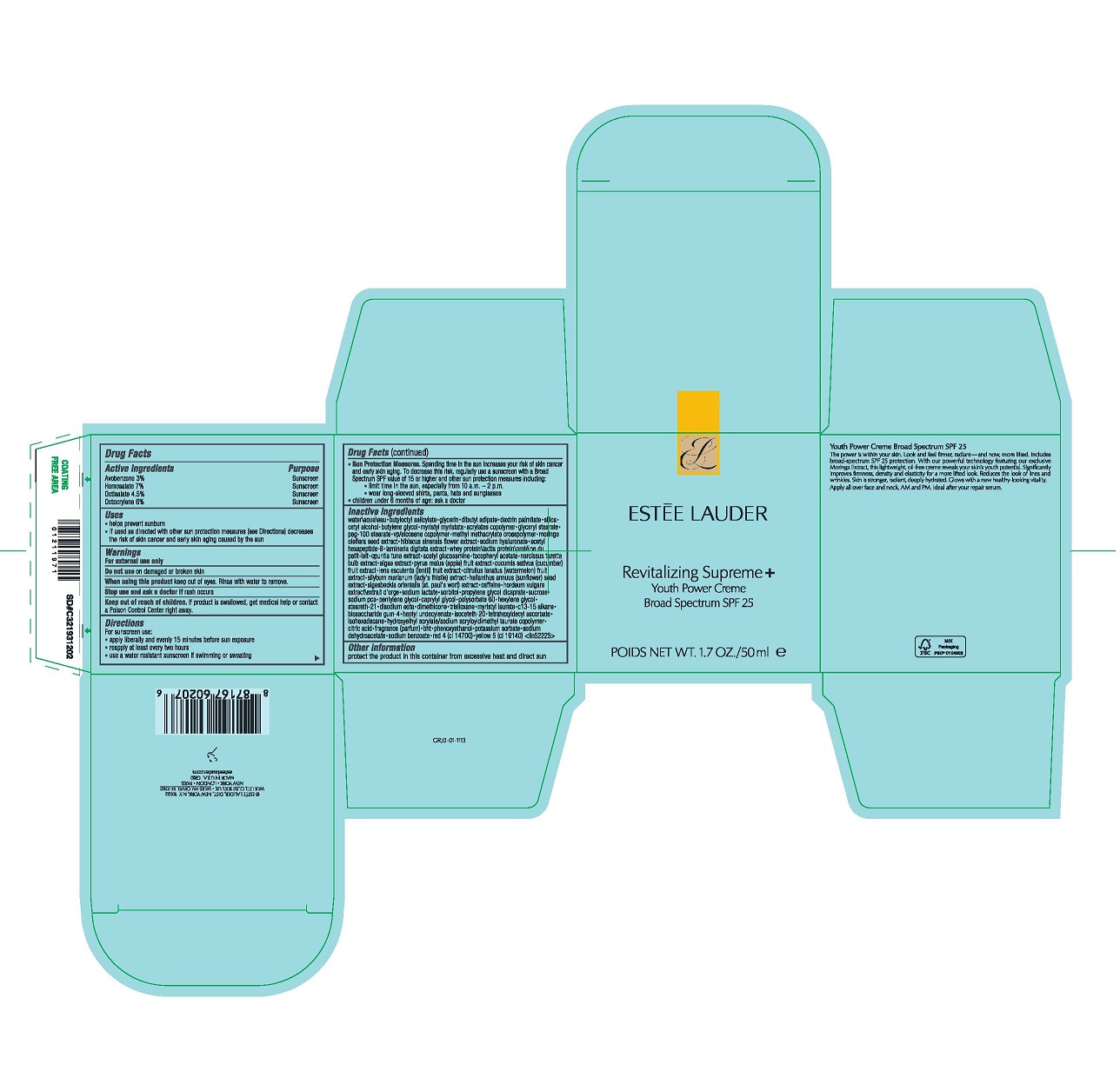
- Active Ingredients
- Purpose
- Uses
- Warnings
-
DOSAGE & ADMINISTRATION
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive Ingredients
WATER\AQUA\EAU,BUTYLOCTYL SALICYLATE,GLYCERIN,DIBUTYL ADIPATE,DEXTRIN PALMITATE,SILICA,CETYL ALCOHOL,BUTYLENE GLYCOL,MYRISTYL MYRISTATE,ACRYLATES COPOLYMER,GLYCERYL STEARATE,PEG-100 STEARATE,VP/EICOSENE COPOLYMER,METHYL METHACRYLATE CROSSPOLYMER,MORINGA OLEIFERA SEED EXTRACT,HIBISCUS SINENSIS FLOWER EXTRACT,SODIUM HYALURONATE,ACETYL HEXAPEPTIDE-8,LAMINARIA DIGITATA EXTRACT,WHEY PROTEIN\LACTIS PROTEIN\PROTÉINE DU PETIT-LAIT,OPUNTIA TUNA EXTRACT,ACETYL GLUCOSAMINE,TOCOPHERYL ACETATE,NARCISSUS TAZETTA BULB EXTRACT,ALGAE EXTRACT,PYRUS MALUS (APPLE) FRUIT EXTRACT,CUCUMIS SATIVUS (CUCUMBER) FRUIT EXTRACT,LENS ESCULENTA (LENTIL) FRUIT EXTRACT,CITRULLUS LANATUS (WATERMELON) FRUIT EXTRACT,SILYBUM MARIANUM (LADY'S THISTLE) EXTRACT,HELIANTHUS ANNUUS (SUNFLOWER) SEED EXTRACT,SIGESBECKIA ORIENTALIS (ST. PAUL'S WORT) EXTRACT,CAFFEINE,HORDEUM VULGARE EXTRACT\EXTRAIT D'ORGE,SODIUM LACTATE,SORBITOL,PROPYLENE GLYCOL DICAPRATE,SUCROSE,SODIUM PCA,PENTYLENE GLYCOL,CAPRYLYL GLYCOL,POLYSORBATE 60,HEXYLENE GLYCOL,STEARETH-21,DISODIUM EDTA,DIMETHICONE,TRISILOXANE,MYRISTYL LAURATE,C13-15 ALKANE,BIOSACCHARIDE GUM-4,HEPTYL UNDECYLENATE,ISOCETETH-20,TETRAHEXYLDECYL ASCORBATE,ISOHEXADECANE,HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER,CITRIC ACID,FRAGRANCE (PARFUM),BHT,PHENOXYETHANOL,POTASSIUM SORBATE,SODIUM DEHYDROACETATE,SODIUM BENZOATE,RED 4 (CI 14700),YELLOW 5 (CI 19140) <ILN52225>
- Other Information
- PDP
-
INGREDIENTS AND APPEARANCE
REVITALIZING SUPREME YOUTH POWER CREME BROAD SPECTRUM SPF 25
avobenzone,homosalate,octisalate, and octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11559-065 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 70 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 60 mg in 1 mL Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CAPRYLYL GLYCOL (UNII: 00YIU5438U) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) POLYSORBATE 60 (UNII: CAL22UVI4M) DIMETHICONE (UNII: 92RU3N3Y1O) SIGESBECKIA ORIENTALIS FLOWERING TOP (UNII: 6UL878YAR7) HEPTYL UNDECYLENATE (UNII: W77QUB6GXO) ISOHEXADECANE (UNII: 918X1OUF1E) PHENOXYETHANOL (UNII: HIE492ZZ3T) WATERMELON (UNII: 231473QB6R) HORDEUM VULGARE WHOLE (UNII: 8JBE478M5Q) SODIUM PYRROLIDONE CARBOXYLATE (UNII: 469OTG57A2) SILYBUM MARIANUM SEED (UNII: U946SH95EE) SUNFLOWER SEED (UNII: R9N3379M4Z) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) HEXYLENE GLYCOL (UNII: KEH0A3F75J) STEARETH-21 (UNII: 53J3F32P58) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) TRISILOXANE (UNII: 9G1ZW13R0G) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) LENS CULINARIS FRUIT (UNII: ZYZ076G9JH) SORBITOL (UNII: 506T60A25R) C13-15 ALKANE (UNII: 114P5I43UJ) FD&C RED NO. 4 (UNII: X3W0AM1JLX) ISOCETETH-20 (UNII: O020065R7Z) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) HIBISCUS MUTABILIS FLOWER (UNII: 2O22799NBH) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) OPUNTIA TUNA FLOWERING TOP (UNII: R4AS8333O2) PORPHYRIDIUM PURPUREUM (UNII: K2P8K2558N) CUCUMBER (UNII: YY7C30VXJT) DEXTRIN PALMITATE (CORN; 20000 MW) (UNII: 89B2BSF9I3) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MYRISTYL MYRISTATE (UNII: 4042ZC00DY) WHEY (UNII: 8617Z5FMF6) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) LAMINARIA DIGITATA (UNII: 15E7C67EE8) APPLE (UNII: B423VGH5S9) HYALURONATE SODIUM (UNII: YSE9PPT4TH) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) PEG-100 STEARATE (UNII: YD01N1999R) MORINGA OLEIFERA SEED (UNII: TIX5482832) METHYL METHACRYLATE/GLYCOL DIMETHACRYLATE CROSSPOLYMER (UNII: EG97988M5Q) CAFFEINE (UNII: 3G6A5W338E) SODIUM LACTATE (UNII: TU7HW0W0QT) PROPYLENE GLYCOL DICAPRATE (UNII: U783H9JHWY) PENTYLENE GLYCOL (UNII: 50C1307PZG) MYRISTYL LAURATE (UNII: 58U0NZN2BT) DIBUTYL ADIPATE (UNII: F4K100DXP3) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) WATER (UNII: 059QF0KO0R) CETYL ALCOHOL (UNII: 936JST6JCN) VINYLPYRROLIDONE/EICOSENE COPOLYMER (UNII: 035MV9S1C3) N-ACETYLGLUCOSAMINE (UNII: V956696549) NARCISSUS TAZETTA BULB (UNII: K17762966S) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11559-065-01 1 in 1 CARTON 01/25/2024 1 75 mL in 1 JAR; Type 0: Not a Combination Product 2 NDC:11559-065-02 1 in 1 CARTON 01/25/2024 2 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/25/2024 Labeler - ESTEE LAUDER INC (005914387) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(11559-065) , pack(11559-065) , label(11559-065) Establishment Name Address ID/FEI Business Operations NORTHTEC LLC 943871157 pack(11559-065) , label(11559-065)

 ESTEE LAUDER
ESTEE LAUDER