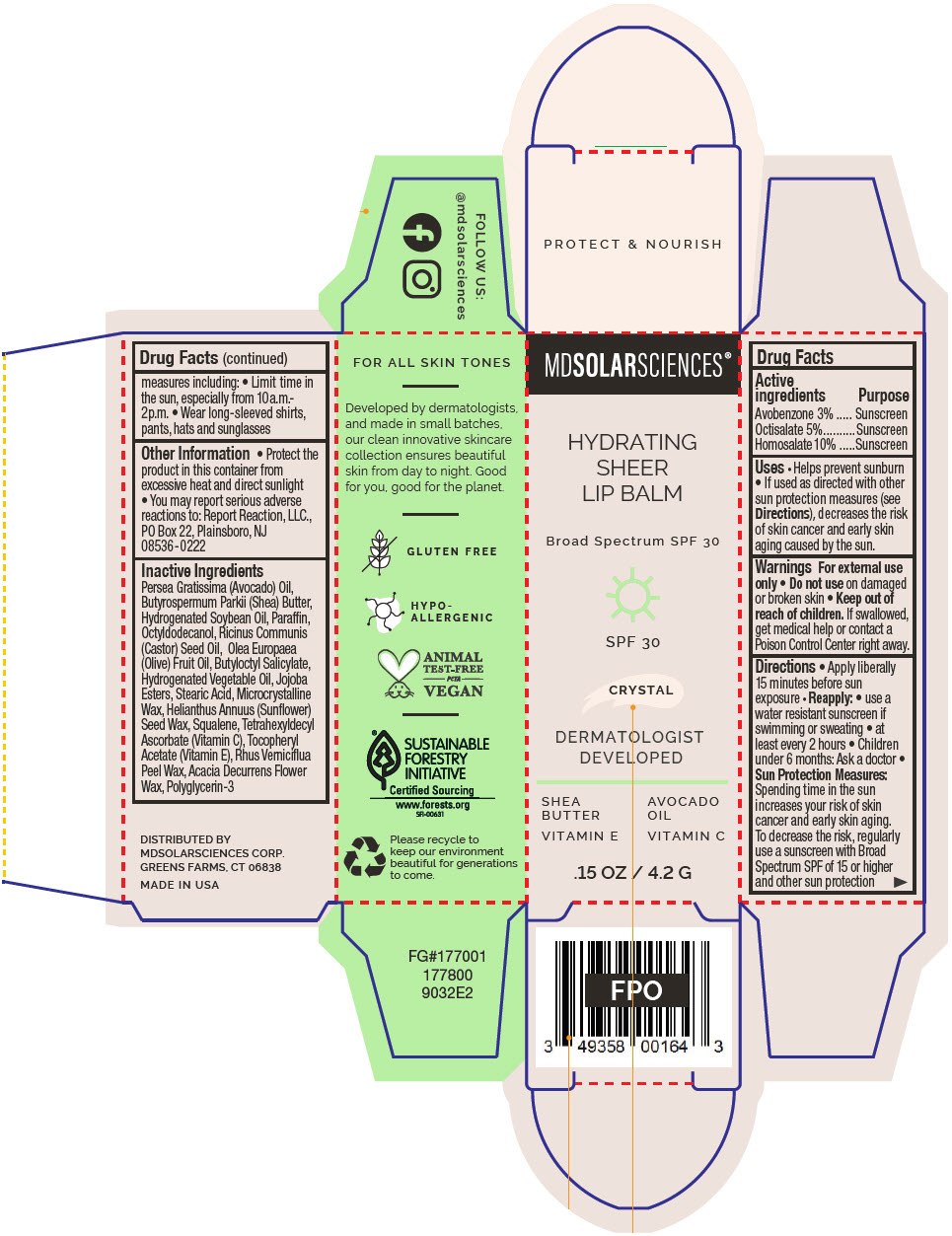
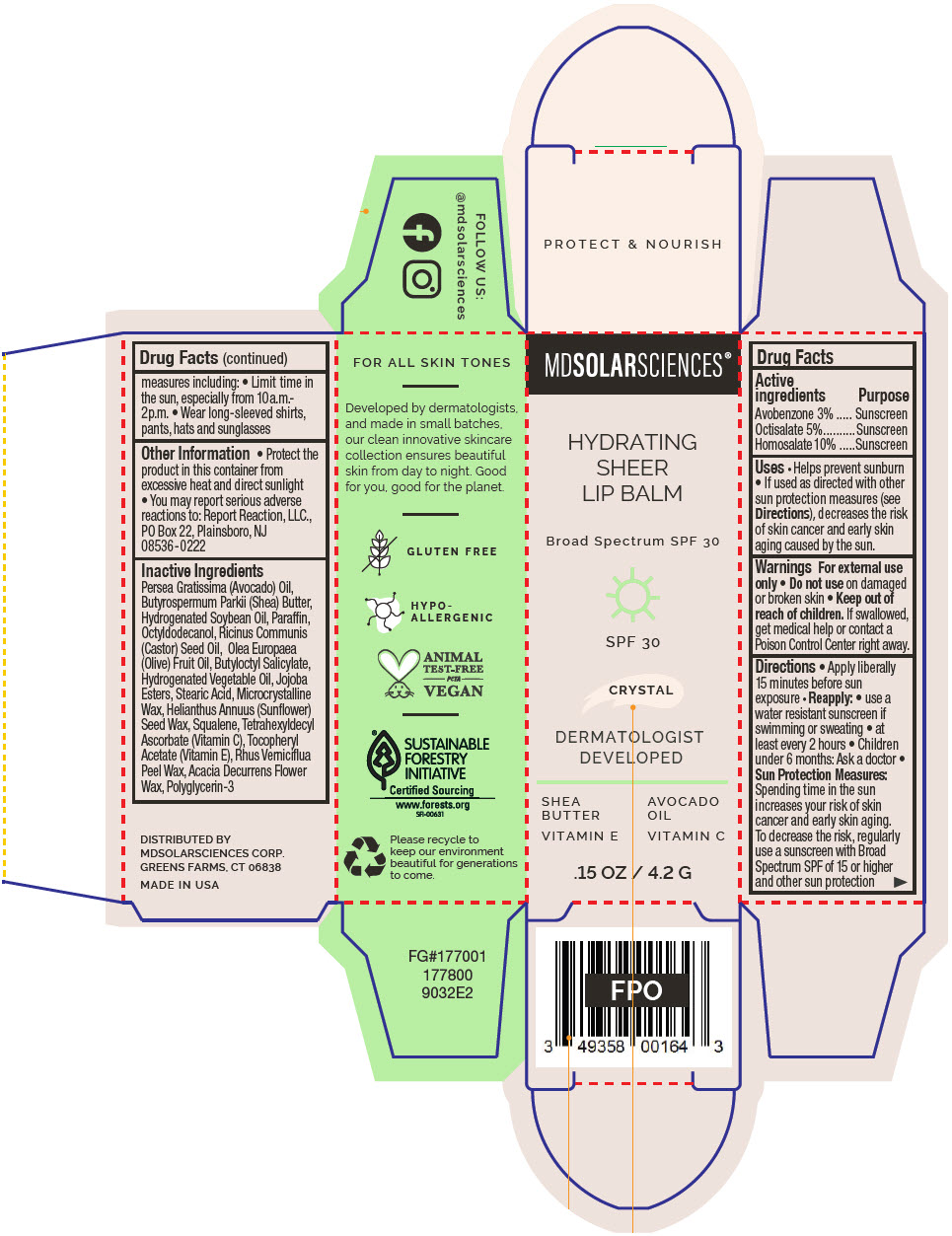
Label: HYDRATING SHEER LIP BALM SPF 30 -CRYSTAL BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN- avobenzone, octisalate, and homosalate lipstick
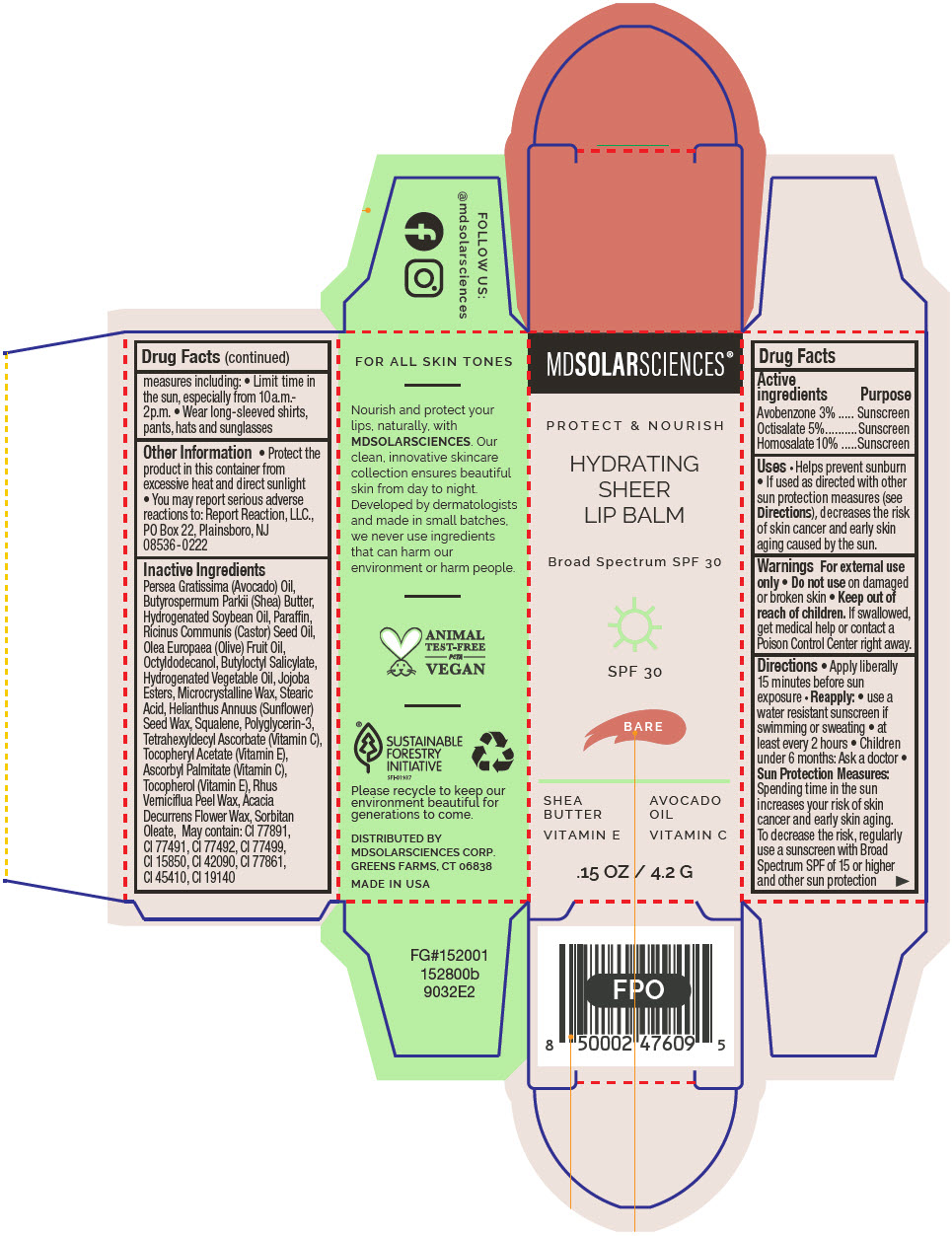
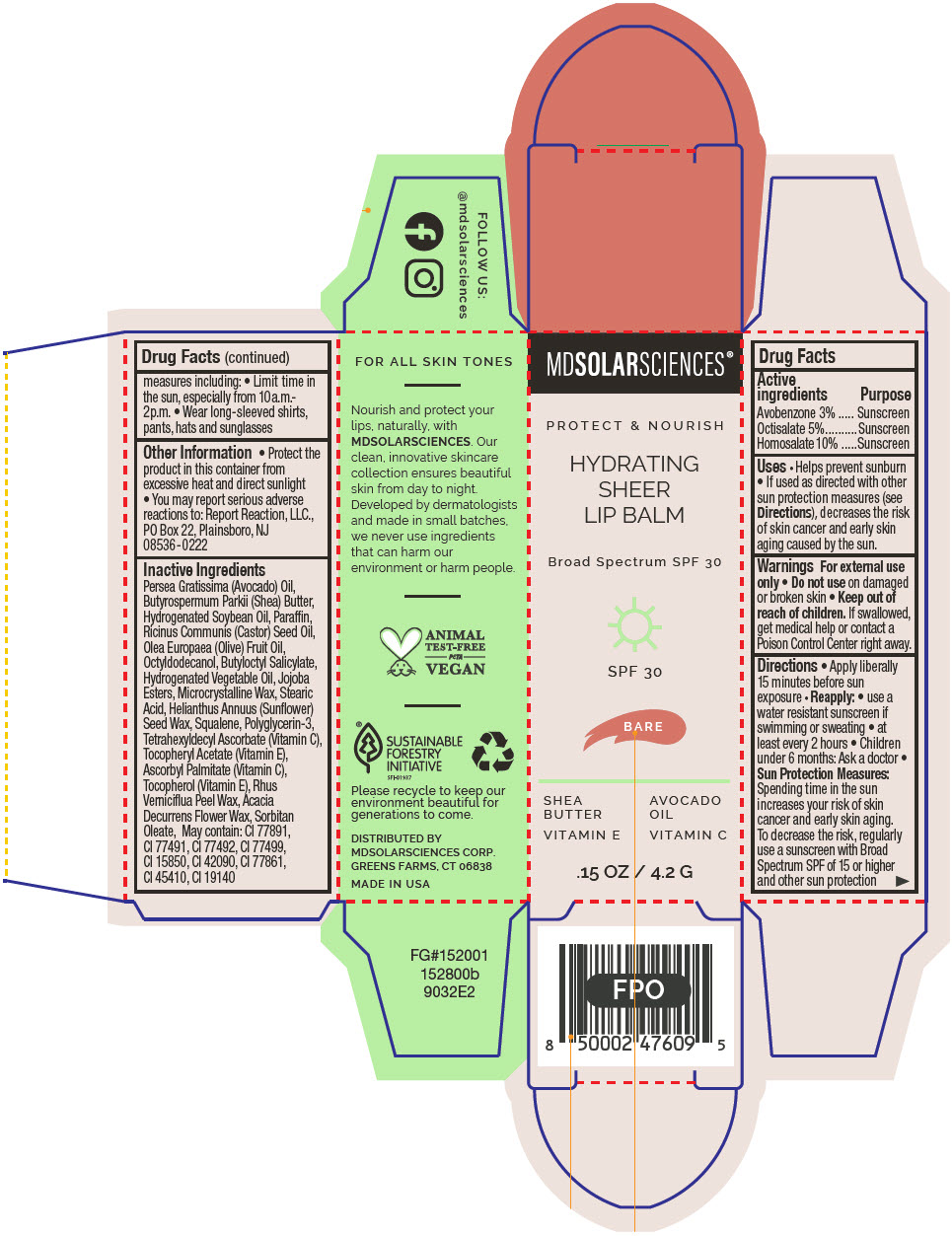
HYDRATING SHEER LIP BALM SPF 30 - BARE BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN- avobenzone, octisalate, and homosalate lipstick
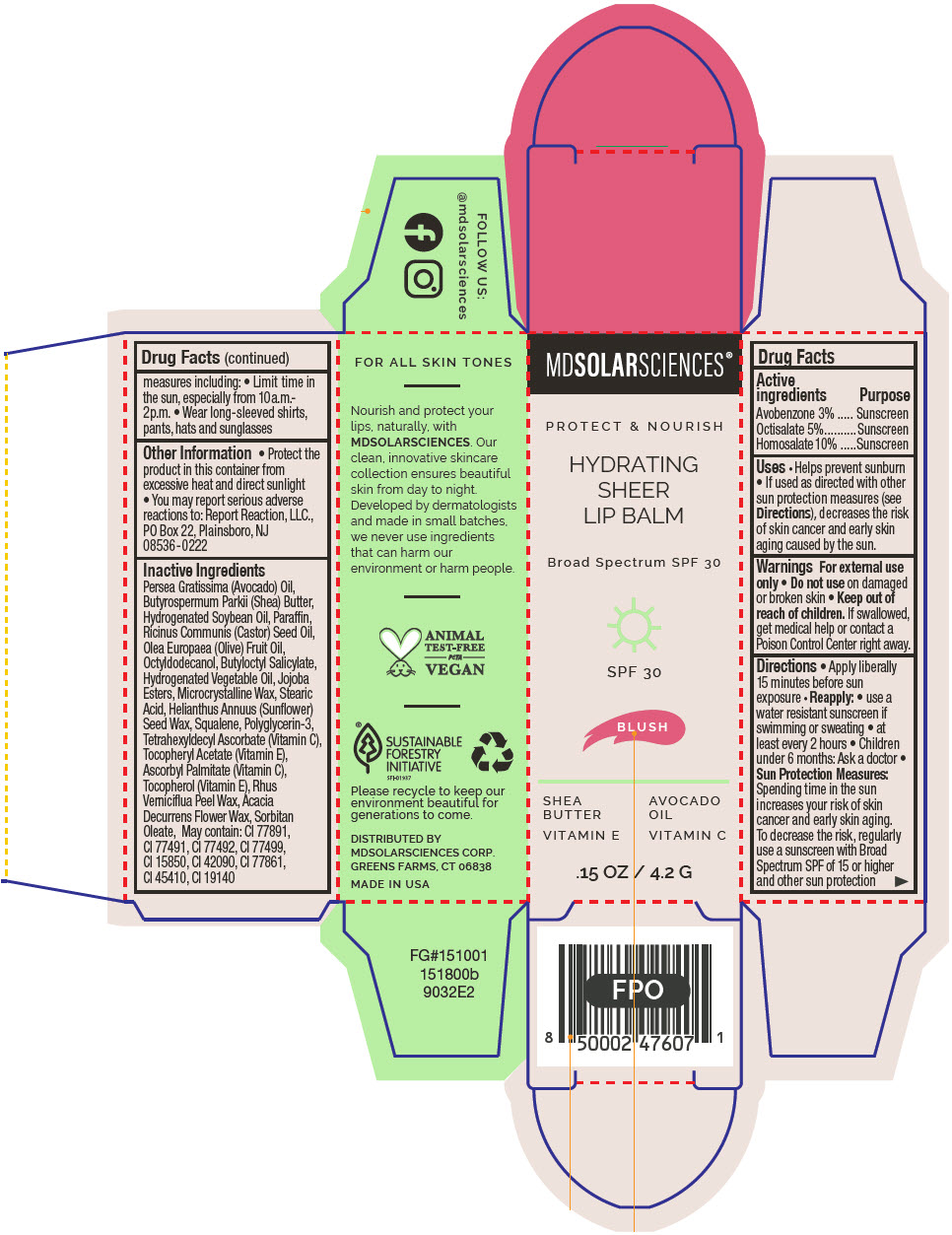
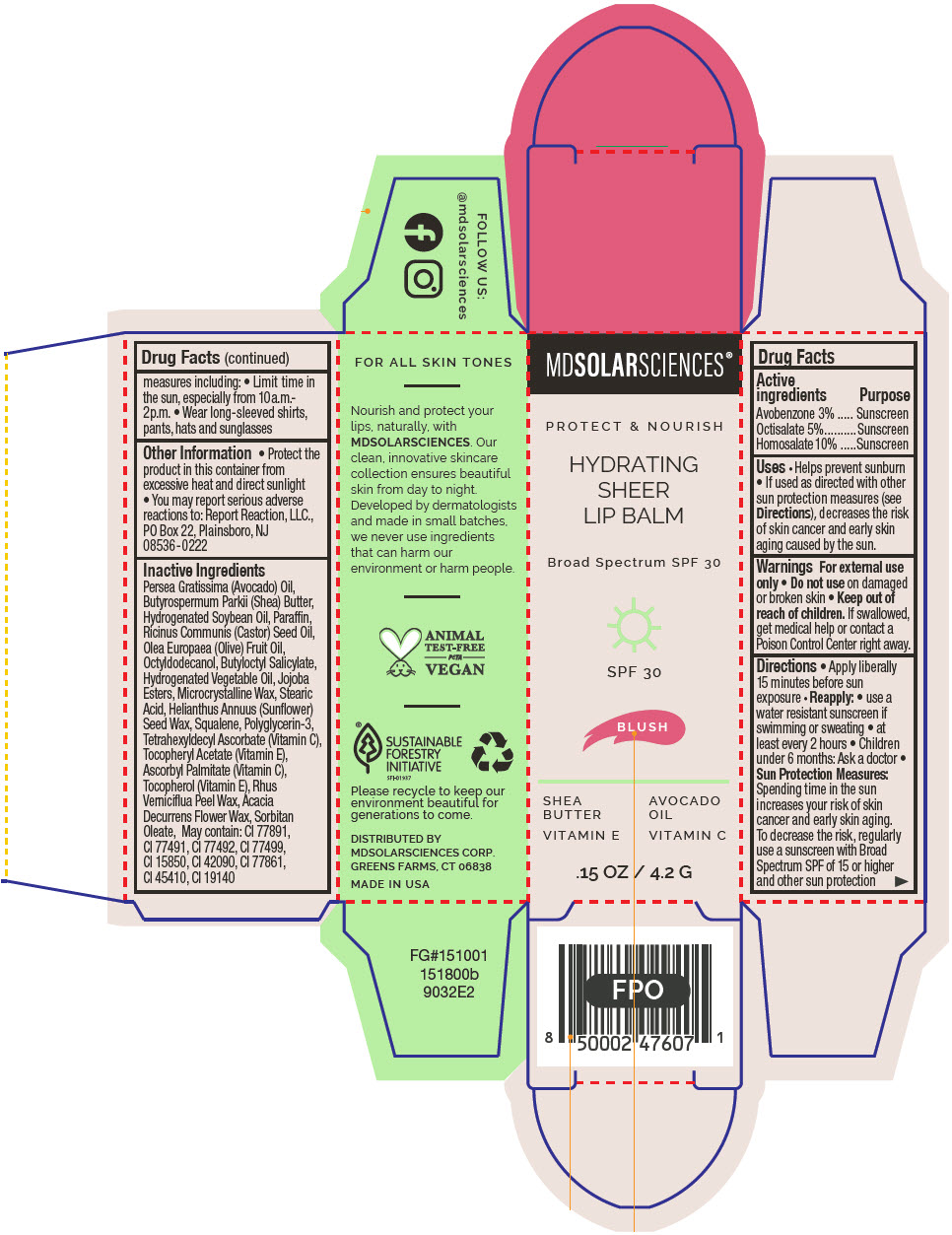
HYDRATING SHEER LIP BALM SPF 30 - BLUSH BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN- avobenzone, octisalate, and homosalate lipstick
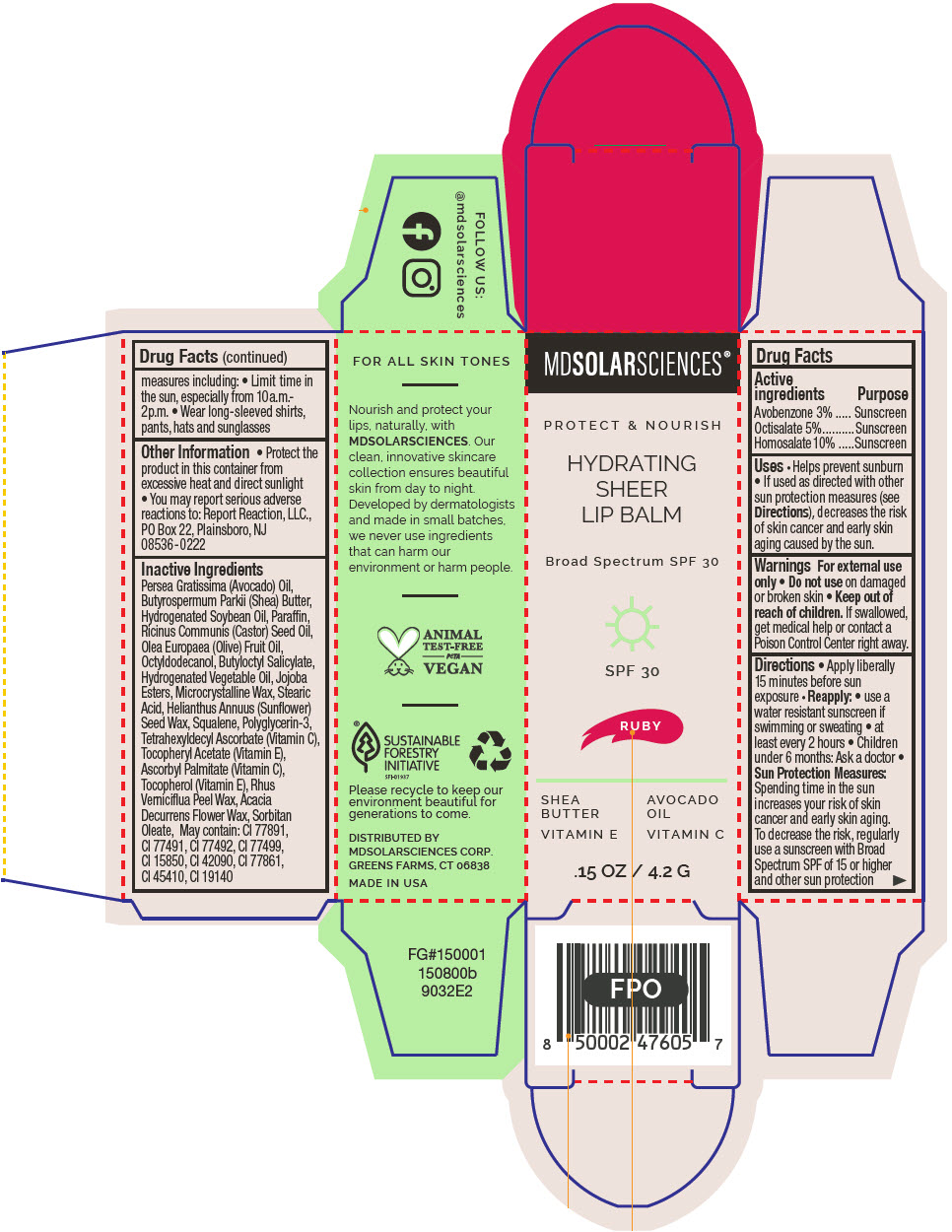
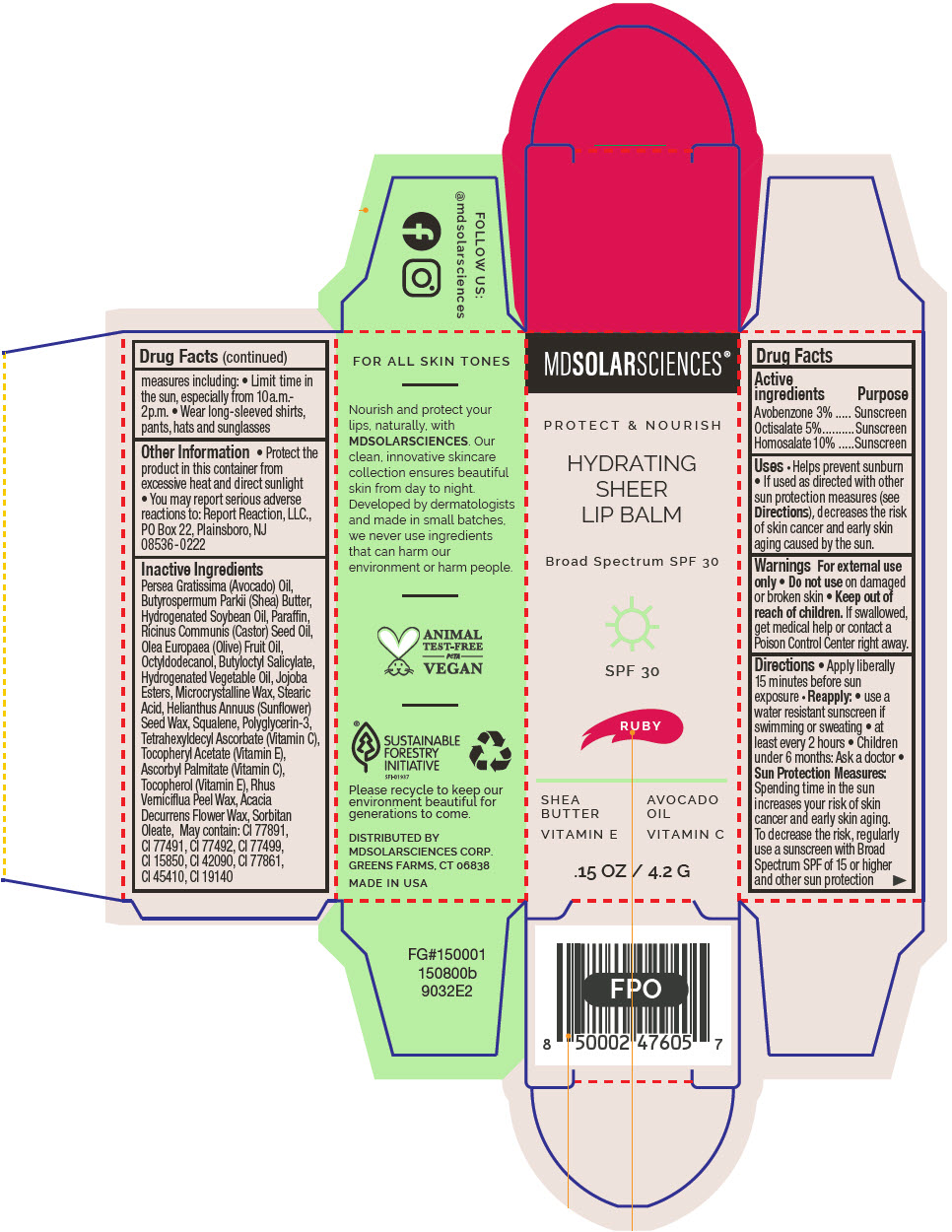
HYDRATING SHEER LIP BALM SPF 30 - RUBY BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN- avobenzone, octisalate, and homosalate lipstick
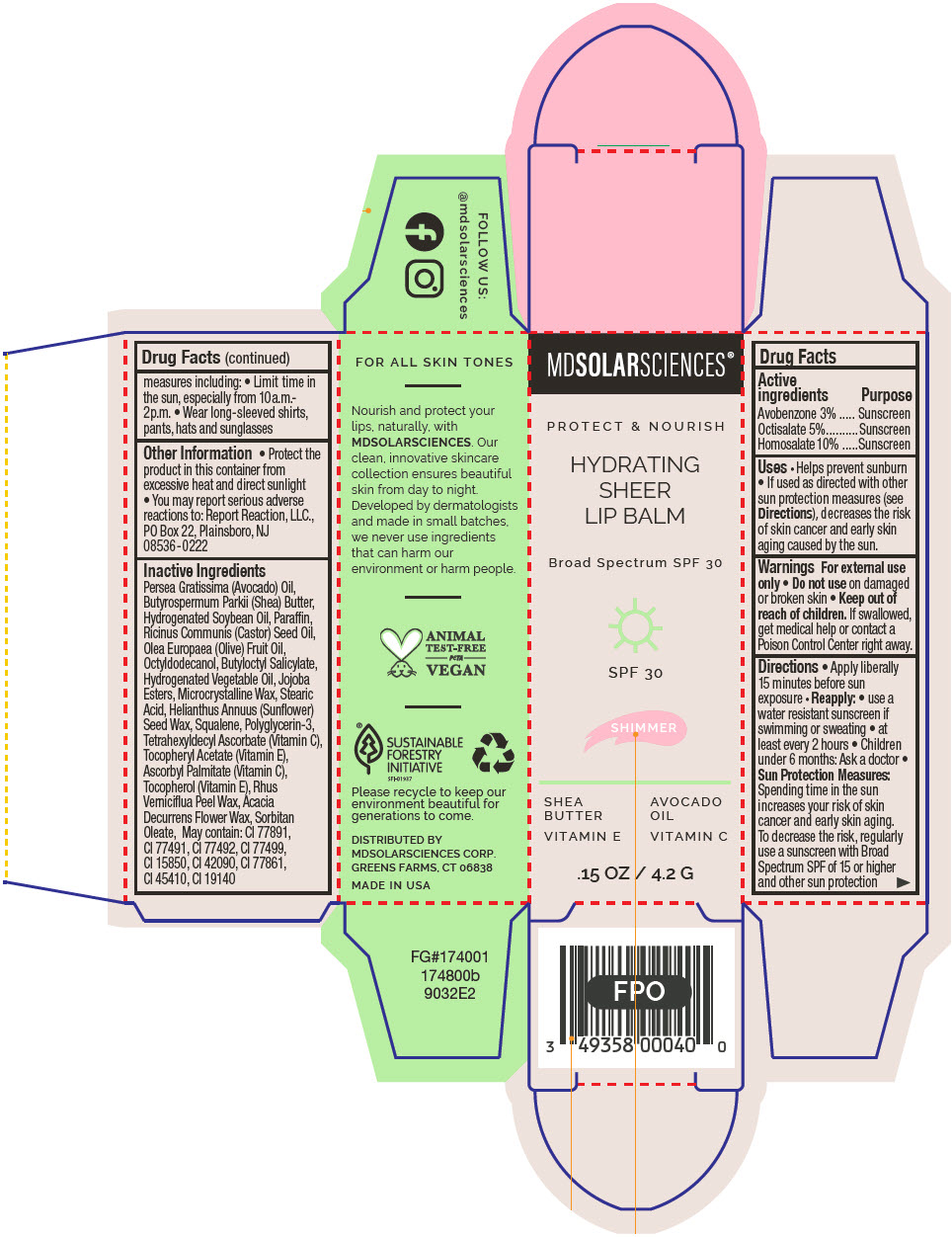
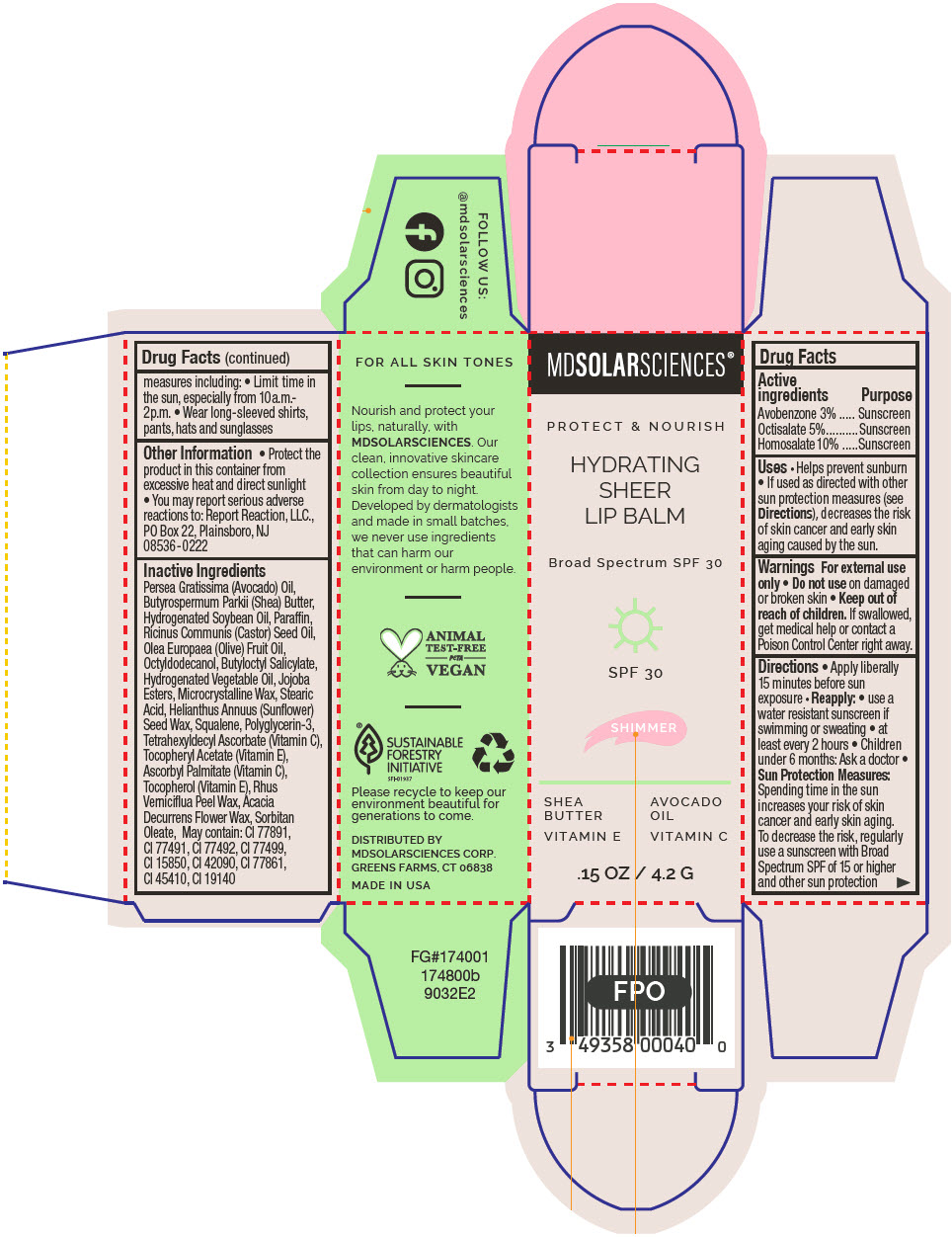
HYDRATING SHEER LIP BALM SPF 30 - SHIMMER BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN- avobenzone, octisalate, and homosalate lipstick
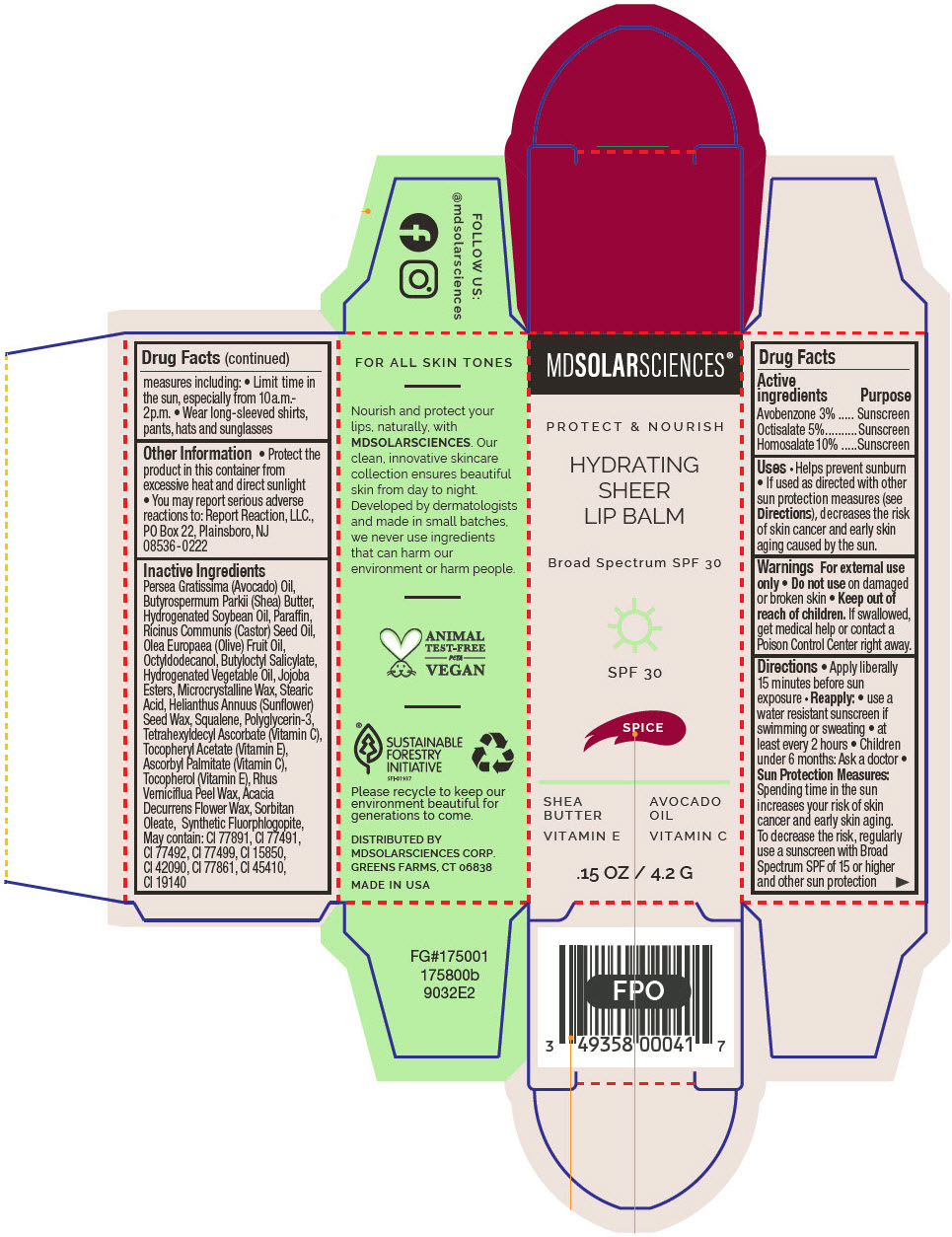
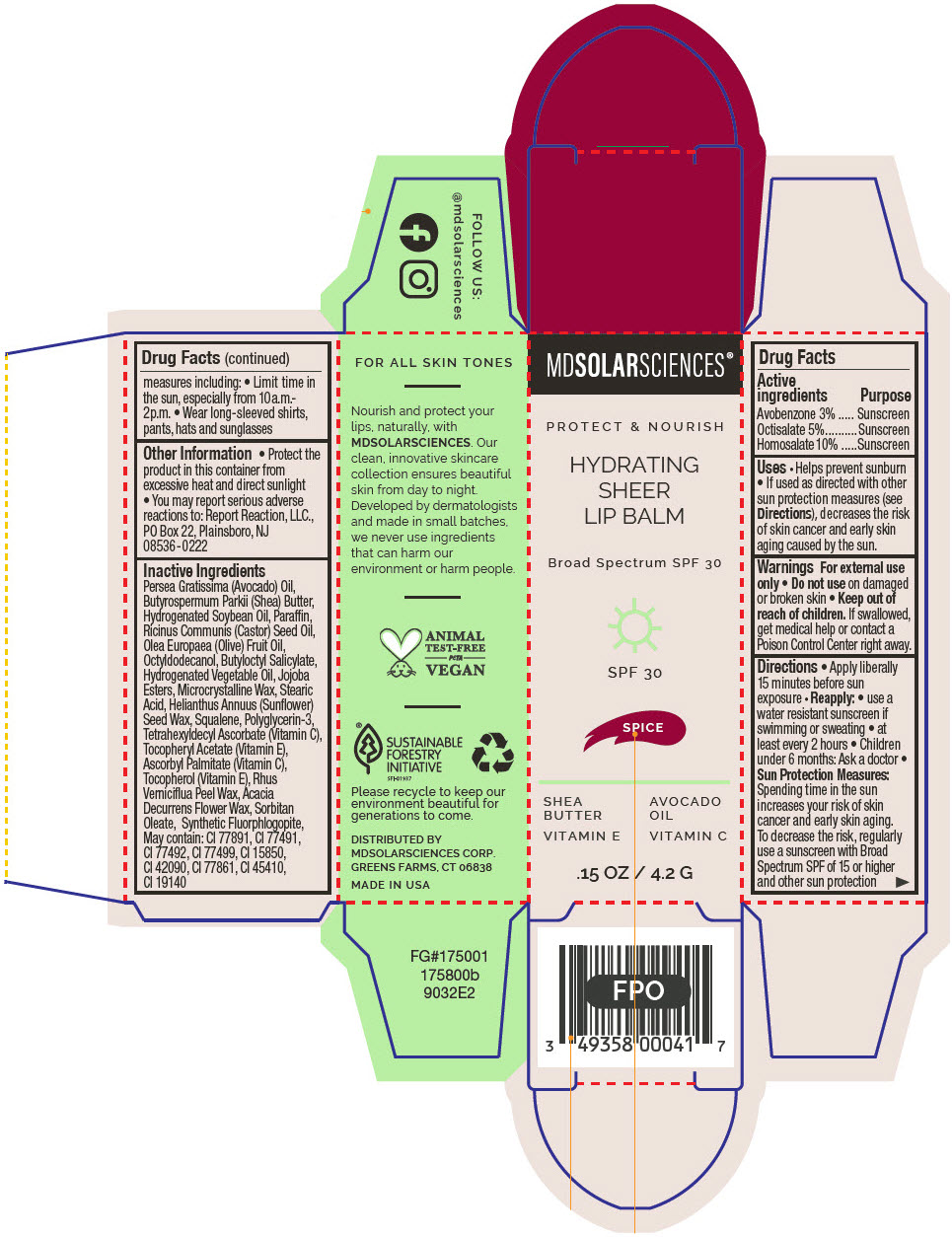
HYDRATING SHEER LIP BALM SPF 30 - SPICE BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN- avobenzone, octisalate, and homosalate lipstick
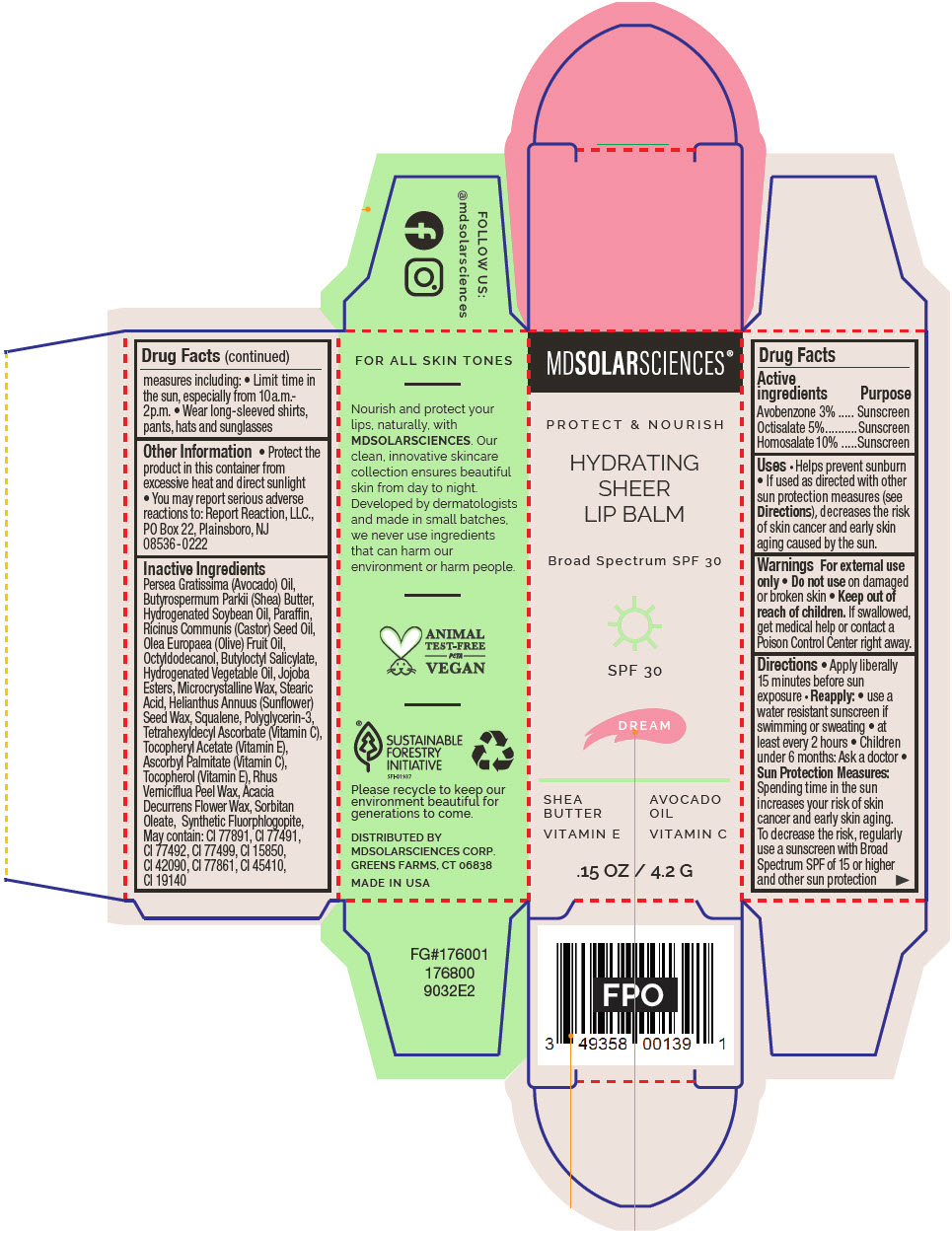
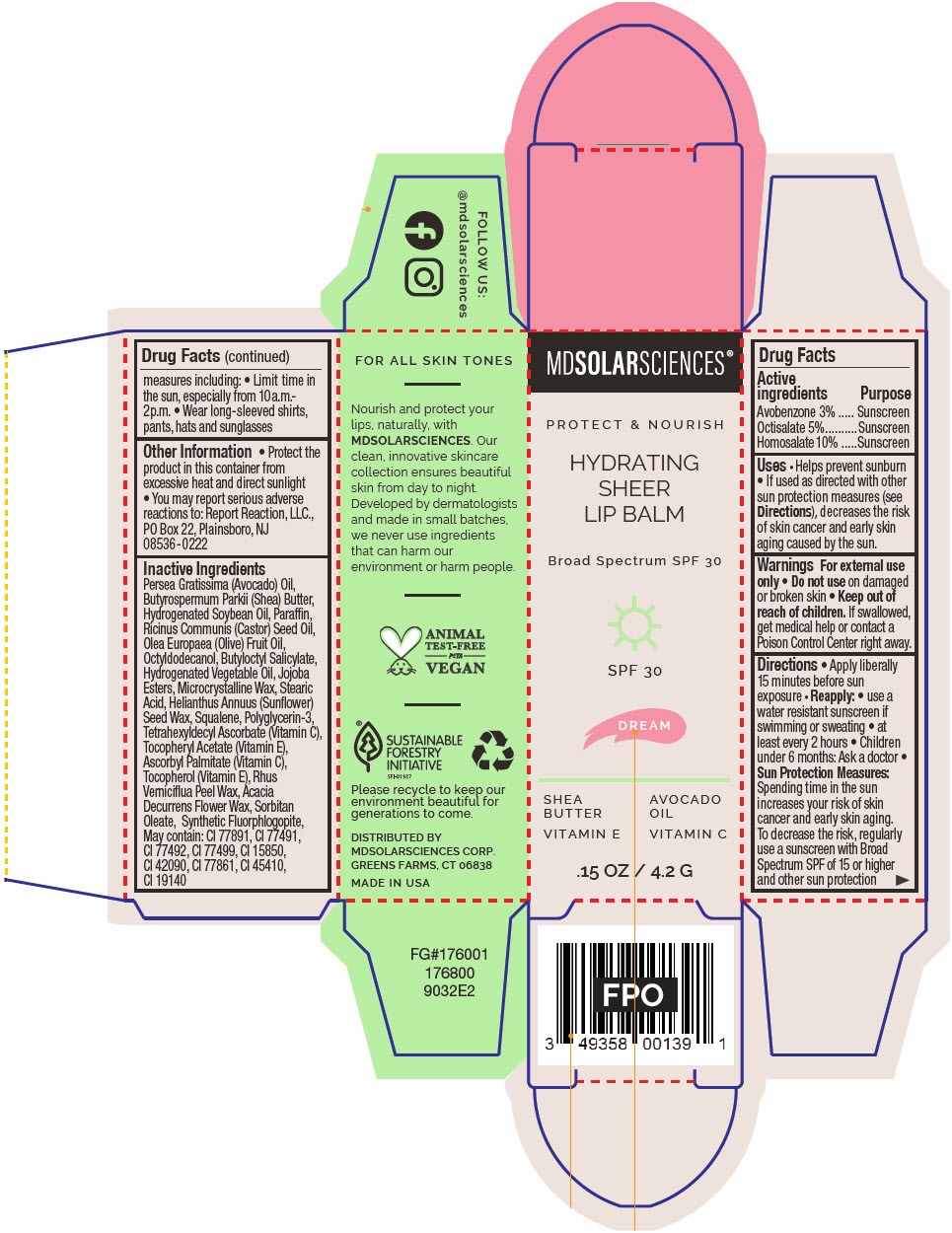
HYDRATING SHEER LIP BALM SPF 30 - DREAM BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN- avobenzone, octisalate, and homosalate lipstick
-
NDC Code(s):
49358-600-01,
49358-601-01,
49358-602-01,
49358-603-01, view more49358-604-01, 49358-605-01, 49358-606-01
- Packager: MDSolarSciences
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 20, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun.
- Warnings
-
Directions
- Apply liberally 15 minutes before sun exposure
-
Reapply:
- use a water resistant sunscreen if swimming or sweating
- at least every 2 hours
- Children under 6 months: Ask a doctor
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging.
To decrease the risk, regularly use a sunscreen with Broad Spectrum SPF of 15 or higher and other sun protection measures including:- Limit time in the sun, especially from 10a.m.- 2p.m.
- Wear long-sleeved shirts, pants, hats and sunglasses
- Other Information
-
Inactive Ingredients
Persea Gratissima (Avocado) Oil, Butyrospermum Parkii (Shea) Butter, Hydrogenated Soybean Oil, Paraffin, Ricinus Communis (Castor) Seed Oil, Olea Europaea (Olive) Fruit Oil, Octyldodecanol, Butyloctyl Salicylate, Hydrogenated Vegetable Oil, Jojoba Esters, Microcrystalline Wax, Stearic Acid, Helianthus Annuus (Sunflower) Seed Wax, Squalene, Polyglycerin-3, Tetrahexyldecyl Ascorbate (Vitamin C), Tocopheryl Acetate (Vitamin E), Ascorbyl Palmitate (Vitamin C), Tocopherol (Vitamin E), Rhus Verniciflua Peel Wax, Acacia Decurrens Flower Wax, Sorbitan Oleate, Synthetic Fluorphlogopite, May contain: CI 77891, CI 77491, CI 77492, CI 77499, CI 15850, CI 42090, CI 77861, CI 45410, CI 19140
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 4.2 G Applicator Carton - Crystal
- PRINCIPAL DISPLAY PANEL - 4.2 G Applicator Carton - Bare
- PRINCIPAL DISPLAY PANEL - 4.2 G Applicator Carton - Blush
- PRINCIPAL DISPLAY PANEL - 4.2 G Applicator Carton - Ruby
- PRINCIPAL DISPLAY PANEL - 4.2 G Applicator Carton - Shimmer
- PRINCIPAL DISPLAY PANEL - 4.2 G Applicator Carton - Spice
- PRINCIPAL DISPLAY PANEL - 4.2 G Applicator Carton - Dream
-
INGREDIENTS AND APPEARANCE
HYDRATING SHEER LIP BALM SPF 30 -CRYSTAL BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN
avobenzone, octisalate, and homosalate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49358-600 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 g Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 100 mg in 1 g Inactive Ingredients Ingredient Name Strength AVOCADO OIL (UNII: 6VNO72PFC1) SHEA BUTTER (UNII: K49155WL9Y) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) Paraffin (UNII: I9O0E3H2ZE) OCTYLDODECANOL (UNII: 461N1O614Y) CASTOR OIL (UNII: D5340Y2I9G) OLIVE OIL (UNII: 6UYK2W1W1E) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CORN OIL (UNII: 8470G57WFM) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) STEARIC ACID (UNII: 4ELV7Z65AP) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) SQUALANE (UNII: GW89575KF9) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOXICODENDRON VERNICIFLUUM FRUIT RIND WAX (UNII: 6RG2461FCH) ACACIA DECURRENS FLOWER WAX (UNII: AU6XZE9IY9) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49358-600-01 1 in 1 CARTON 07/01/2023 1 4.2 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/01/2023 HYDRATING SHEER LIP BALM SPF 30 - BARE BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN
avobenzone, octisalate, and homosalate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49358-601 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 g Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 100 mg in 1 g Inactive Ingredients Ingredient Name Strength AVOCADO OIL (UNII: 6VNO72PFC1) SHEA BUTTER (UNII: K49155WL9Y) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) Paraffin (UNII: I9O0E3H2ZE) CASTOR OIL (UNII: D5340Y2I9G) OLIVE OIL (UNII: 6UYK2W1W1E) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CORN OIL (UNII: 8470G57WFM) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) STEARIC ACID (UNII: 4ELV7Z65AP) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) SQUALANE (UNII: GW89575KF9) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOXICODENDRON VERNICIFLUUM FRUIT RIND WAX (UNII: 6RG2461FCH) ACACIA DECURRENS FLOWER WAX (UNII: AU6XZE9IY9) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain TITANIUM DIOXIDE (UNII: 15FIX9V2JP) May contain FERRIC OXIDE RED (UNII: 1K09F3G675) May contain FERRIC OXIDE YELLOW (UNII: EX438O2MRT) May contain FERROSOFERRIC OXIDE (UNII: XM0M87F357) May contain D&C RED NO. 6 (UNII: 481744AI4O) May contain FD&C BLUE NO. 1 (UNII: H3R47K3TBD) May contain STANNIC OXIDE (UNII: KM7N50LOS6) May contain D&C RED NO. 28 (UNII: 767IP0Y5NH) May contain FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color BROWN Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49358-601-01 1 in 1 CARTON 07/01/2023 1 4.2 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/01/2023 HYDRATING SHEER LIP BALM SPF 30 - BLUSH BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN
avobenzone, octisalate, and homosalate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49358-602 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 g Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 100 mg in 1 g Inactive Ingredients Ingredient Name Strength AVOCADO OIL (UNII: 6VNO72PFC1) SHEA BUTTER (UNII: K49155WL9Y) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) Paraffin (UNII: I9O0E3H2ZE) CASTOR OIL (UNII: D5340Y2I9G) OLIVE OIL (UNII: 6UYK2W1W1E) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CORN OIL (UNII: 8470G57WFM) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) STEARIC ACID (UNII: 4ELV7Z65AP) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) SQUALANE (UNII: GW89575KF9) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOXICODENDRON VERNICIFLUUM FRUIT RIND WAX (UNII: 6RG2461FCH) ACACIA DECURRENS FLOWER WAX (UNII: AU6XZE9IY9) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain TITANIUM DIOXIDE (UNII: 15FIX9V2JP) May contain FERRIC OXIDE RED (UNII: 1K09F3G675) May contain FERRIC OXIDE YELLOW (UNII: EX438O2MRT) May contain FERROSOFERRIC OXIDE (UNII: XM0M87F357) May contain D&C RED NO. 6 (UNII: 481744AI4O) May contain FD&C BLUE NO. 1 (UNII: H3R47K3TBD) May contain STANNIC OXIDE (UNII: KM7N50LOS6) May contain D&C RED NO. 28 (UNII: 767IP0Y5NH) May contain FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color PINK Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49358-602-01 1 in 1 CARTON 07/01/2023 1 4.2 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/01/2023 HYDRATING SHEER LIP BALM SPF 30 - RUBY BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN
avobenzone, octisalate, and homosalate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49358-603 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 g Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 100 mg in 1 g Inactive Ingredients Ingredient Name Strength AVOCADO OIL (UNII: 6VNO72PFC1) SHEA BUTTER (UNII: K49155WL9Y) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) Paraffin (UNII: I9O0E3H2ZE) CASTOR OIL (UNII: D5340Y2I9G) OLIVE OIL (UNII: 6UYK2W1W1E) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CORN OIL (UNII: 8470G57WFM) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) STEARIC ACID (UNII: 4ELV7Z65AP) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) SQUALANE (UNII: GW89575KF9) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOXICODENDRON VERNICIFLUUM FRUIT RIND WAX (UNII: 6RG2461FCH) ACACIA DECURRENS FLOWER WAX (UNII: AU6XZE9IY9) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain TITANIUM DIOXIDE (UNII: 15FIX9V2JP) May contain FERRIC OXIDE RED (UNII: 1K09F3G675) May contain FERRIC OXIDE YELLOW (UNII: EX438O2MRT) May contain FERROSOFERRIC OXIDE (UNII: XM0M87F357) May contain D&C RED NO. 6 (UNII: 481744AI4O) May contain FD&C BLUE NO. 1 (UNII: H3R47K3TBD) May contain STANNIC OXIDE (UNII: KM7N50LOS6) May contain D&C RED NO. 28 (UNII: 767IP0Y5NH) May contain FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color RED Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49358-603-01 1 in 1 CARTON 07/01/2023 1 4.2 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/01/2023 HYDRATING SHEER LIP BALM SPF 30 - SHIMMER BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN
avobenzone, octisalate, and homosalate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49358-604 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 g Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 100 mg in 1 g Inactive Ingredients Ingredient Name Strength AVOCADO OIL (UNII: 6VNO72PFC1) SHEA BUTTER (UNII: K49155WL9Y) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) Paraffin (UNII: I9O0E3H2ZE) CASTOR OIL (UNII: D5340Y2I9G) OLIVE OIL (UNII: 6UYK2W1W1E) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CORN OIL (UNII: 8470G57WFM) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) STEARIC ACID (UNII: 4ELV7Z65AP) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) SQUALANE (UNII: GW89575KF9) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOXICODENDRON VERNICIFLUUM FRUIT RIND WAX (UNII: 6RG2461FCH) ACACIA DECURRENS FLOWER WAX (UNII: AU6XZE9IY9) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain TITANIUM DIOXIDE (UNII: 15FIX9V2JP) May contain FERRIC OXIDE RED (UNII: 1K09F3G675) May contain FERRIC OXIDE YELLOW (UNII: EX438O2MRT) May contain FERROSOFERRIC OXIDE (UNII: XM0M87F357) May contain D&C RED NO. 6 (UNII: 481744AI4O) May contain FD&C BLUE NO. 1 (UNII: H3R47K3TBD) May contain STANNIC OXIDE (UNII: KM7N50LOS6) May contain D&C RED NO. 28 (UNII: 767IP0Y5NH) May contain FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color PINK Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49358-604-01 1 in 1 CARTON 07/01/2023 1 4.2 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/01/2023 HYDRATING SHEER LIP BALM SPF 30 - SPICE BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN
avobenzone, octisalate, and homosalate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49358-605 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 g Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 100 mg in 1 g Inactive Ingredients Ingredient Name Strength AVOCADO OIL (UNII: 6VNO72PFC1) SHEA BUTTER (UNII: K49155WL9Y) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) Paraffin (UNII: I9O0E3H2ZE) CASTOR OIL (UNII: D5340Y2I9G) OLIVE OIL (UNII: 6UYK2W1W1E) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CORN OIL (UNII: 8470G57WFM) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) STEARIC ACID (UNII: 4ELV7Z65AP) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) SQUALANE (UNII: GW89575KF9) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOXICODENDRON VERNICIFLUUM FRUIT RIND WAX (UNII: 6RG2461FCH) ACACIA DECURRENS FLOWER WAX (UNII: AU6XZE9IY9) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain TITANIUM DIOXIDE (UNII: 15FIX9V2JP) May contain FERRIC OXIDE RED (UNII: 1K09F3G675) May contain FERRIC OXIDE YELLOW (UNII: EX438O2MRT) May contain FERROSOFERRIC OXIDE (UNII: XM0M87F357) May contain D&C RED NO. 6 (UNII: 481744AI4O) May contain FD&C BLUE NO. 1 (UNII: H3R47K3TBD) May contain STANNIC OXIDE (UNII: KM7N50LOS6) May contain D&C RED NO. 28 (UNII: 767IP0Y5NH) May contain FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color RED Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49358-605-01 1 in 1 CARTON 07/01/2023 1 4.2 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/01/2023 HYDRATING SHEER LIP BALM SPF 30 - DREAM BROAD SPECTRUM SPF 30 UVA-UVB SUNSCREEN
avobenzone, octisalate, and homosalate lipstickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:49358-606 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Avobenzone (UNII: G63QQF2NOX) (Avobenzone - UNII:G63QQF2NOX) Avobenzone 30 mg in 1 g Octisalate (UNII: 4X49Y0596W) (Octisalate - UNII:4X49Y0596W) Octisalate 50 mg in 1 g Homosalate (UNII: V06SV4M95S) (Homosalate - UNII:V06SV4M95S) Homosalate 100 mg in 1 g Inactive Ingredients Ingredient Name Strength AVOCADO OIL (UNII: 6VNO72PFC1) SHEA BUTTER (UNII: K49155WL9Y) HYDROGENATED SOYBEAN OIL (UNII: A2M91M918C) Paraffin (UNII: I9O0E3H2ZE) CASTOR OIL (UNII: D5340Y2I9G) OLIVE OIL (UNII: 6UYK2W1W1E) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) CORN OIL (UNII: 8470G57WFM) HYDROGENATED JOJOBA OIL, RANDOMIZED (UNII: Q47ST02F58) MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) STEARIC ACID (UNII: 4ELV7Z65AP) HELIANTHUS ANNUUS SEED WAX (UNII: 42DG15CHXV) SQUALANE (UNII: GW89575KF9) POLYGLYCERIN-3 (UNII: 4A0NCJ6RD6) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TOCOPHEROL (UNII: R0ZB2556P8) ASCORBYL PALMITATE (UNII: QN83US2B0N) TOXICODENDRON VERNICIFLUUM FRUIT RIND WAX (UNII: 6RG2461FCH) ACACIA DECURRENS FLOWER WAX (UNII: AU6XZE9IY9) SORBITAN MONOOLEATE (UNII: 06XEA2VD56) MAGNESIUM POTASSIUM ALUMINOSILICATE FLUORIDE (UNII: YK3DC63Y5M) Other Ingredients Ingredient Kind Ingredient Name Quantity May contain TITANIUM DIOXIDE (UNII: 15FIX9V2JP) May contain FERRIC OXIDE RED (UNII: 1K09F3G675) May contain FERRIC OXIDE YELLOW (UNII: EX438O2MRT) May contain FERROSOFERRIC OXIDE (UNII: XM0M87F357) May contain D&C RED NO. 6 (UNII: 481744AI4O) May contain FD&C BLUE NO. 1 (UNII: H3R47K3TBD) May contain STANNIC OXIDE (UNII: KM7N50LOS6) May contain D&C RED NO. 28 (UNII: 767IP0Y5NH) May contain FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Product Characteristics Color PINK Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:49358-606-01 1 in 1 CARTON 07/01/2023 1 4.2 g in 1 APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 07/01/2023 Labeler - MDSolarSciences (013647301)