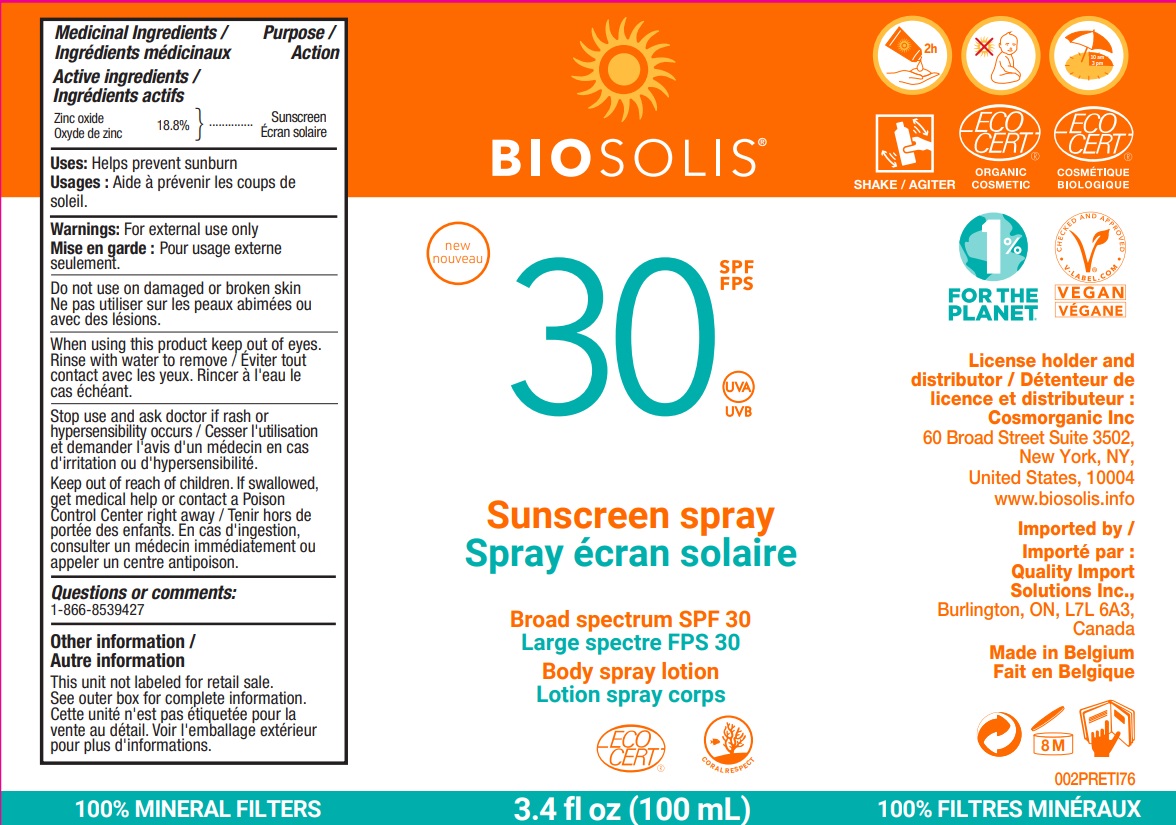
Label: BIOSOLIS SUNSCREEN BROADSPECTRUM SPF30- zinc oxide cream
- NDC Code(s): 61296-015-00
- Packager: Pro Vera SA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- Sun protection measures: Spending time in the sun increases your risk of skin cancer and early skin aging.To decrease this risk, regularly use a sunscreen with aBroad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 3 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- reapply
- after 40 minutes swimming or sweating
- immediately after towel drying
- at least every 2 hours
- children under 6 months of age: Ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- Package Labeling:61296-015-00
-
INGREDIENTS AND APPEARANCE
BIOSOLIS SUNSCREEN BROADSPECTRUM SPF30
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61296-015 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 188 mg in 1 mL Inactive Ingredients Ingredient Name Strength LEVOMENOL (UNII: 24WE03BX2T) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) COCONUT OIL (UNII: Q9L0O73W7L) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) HYDROGENATED COCONUT OIL (UNII: JY81OXM1OM) PONGAMIA PINNATA SEED (UNII: C2BRV53B1V) LIMONENE, (+)- (UNII: GFD7C86Q1W) LINALOOL, (+/-)- (UNII: D81QY6I88E) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) SESAME OIL (UNII: QX10HYY4QV) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61296-015-00 1 in 1 BOX 01/07/2022 04/30/2025 1 100 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/07/2022 04/30/2025 Labeler - Pro Vera SA (375713286) Registrant - Pro Vera SA (375713286)