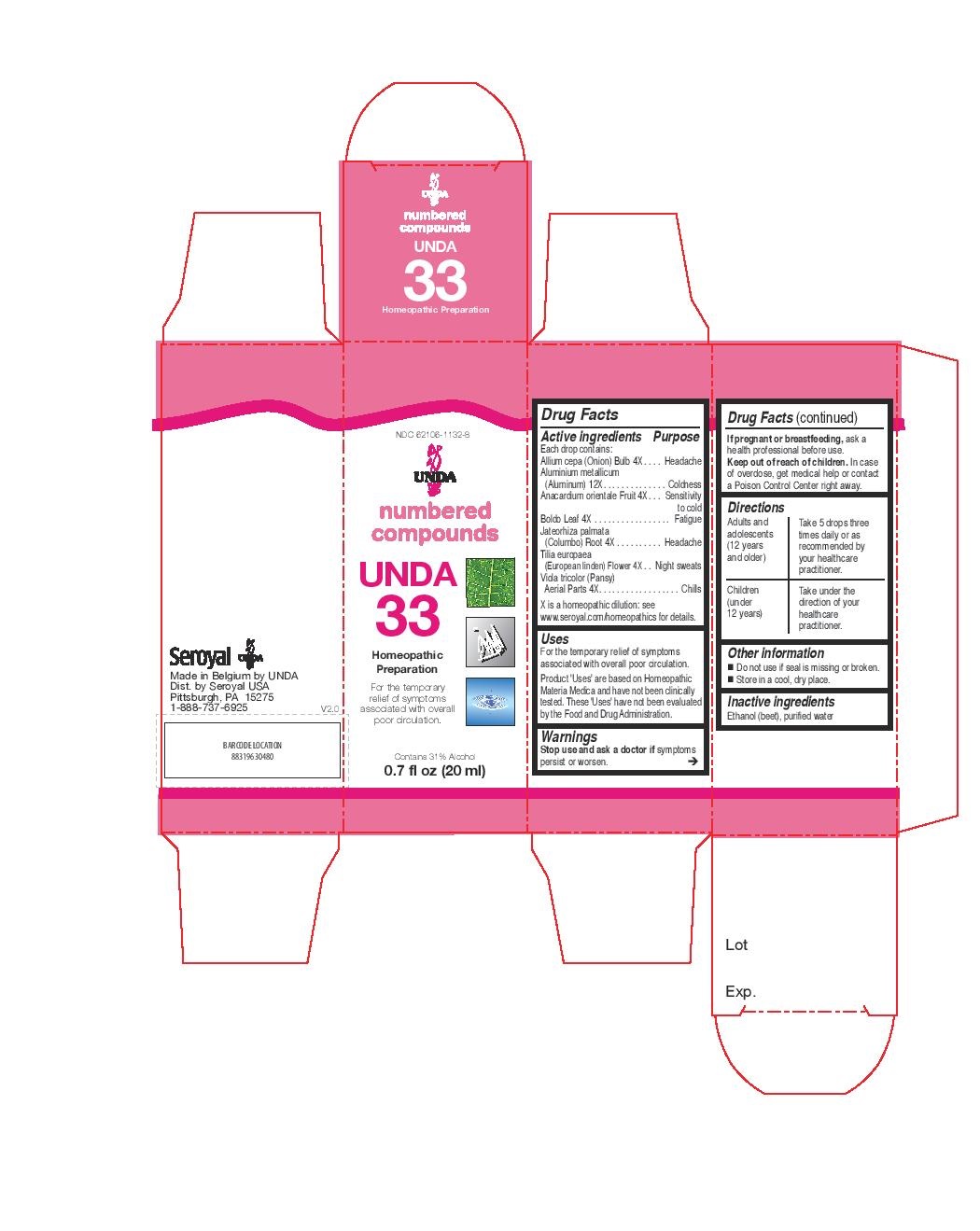
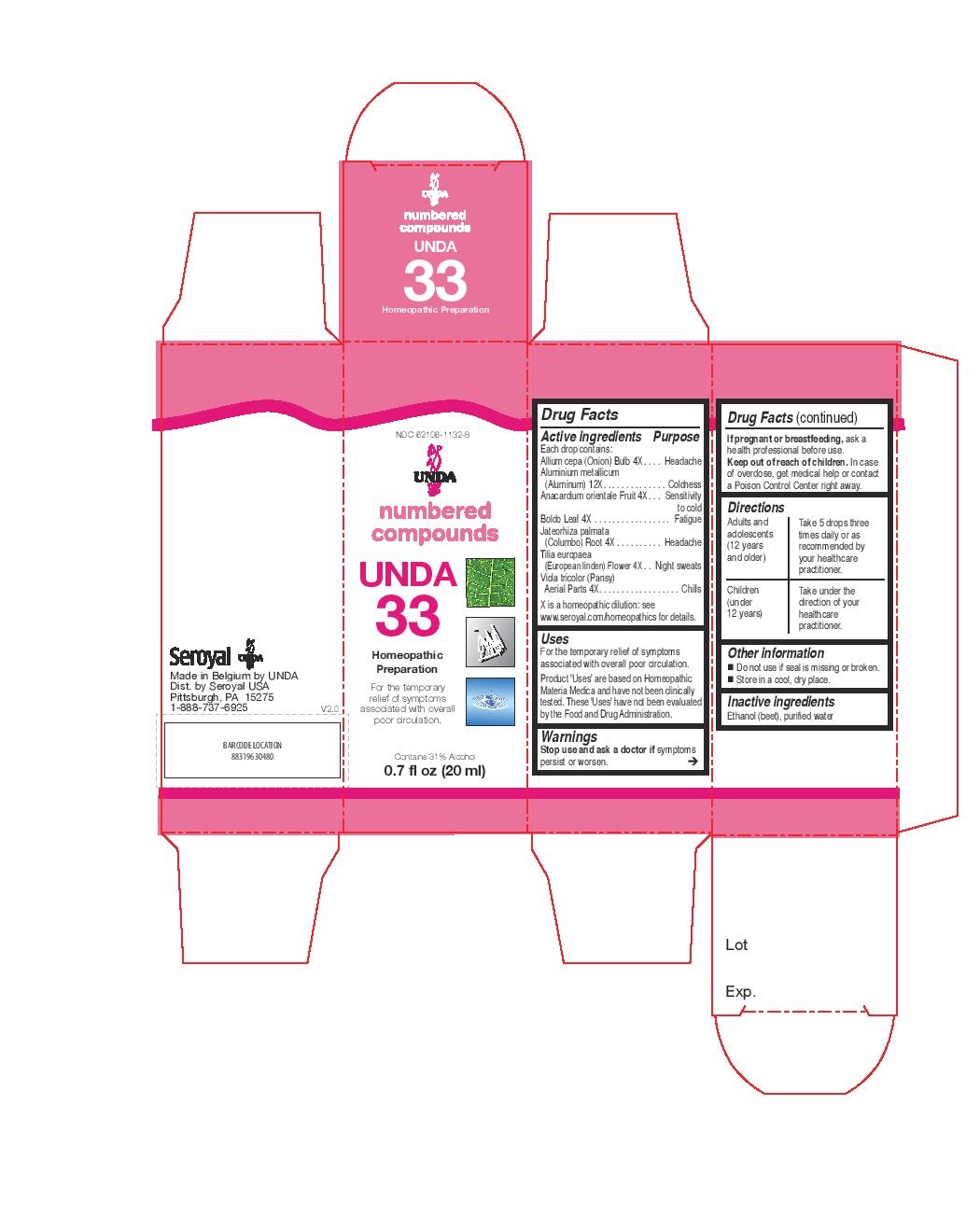
Label: UNDA 33- allium cepa, tilia europaea, jateorhiza palmata, viola tricolor, boldo leaf, anacardium orientale frui, aluminium metallicum liquid
- NDC Code(s): 62106-1132-8
- Packager: Seroyal USA
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated November 9, 2022
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- OVERDOSAGE
- ASK DOCTOR
- INACTIVE INGREDIENT
- OTHER SAFETY INFORMATION
- DOSAGE & ADMINISTRATION
-
INDICATIONS & USAGE
Uses
For the temporary relief of symptoms associated with overall poor circulation.Directions
Adults and adolescents (12 years and older)Take 5 drops three times daily or as recommended by your healthcare practitioner.
Children (under 12 years)
Take under the direction of your healthcare practitioner. - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
UNDA 33
allium cepa, tilia europaea, jateorhiza palmata, viola tricolor, boldo leaf, anacardium orientale frui, aluminium metallicum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:62106-1132 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ONION (UNII: 492225Q21H) (ONION - UNII:492225Q21H) ONION 4 [hp_X] in 20 mL TILIA X EUROPAEA FLOWER (UNII: NHV2K1OUDH) (TILIA X EUROPAEA FLOWER - UNII:NHV2K1OUDH) TILIA X EUROPAEA FLOWER 4 [hp_X] in 20 mL JATEORHIZA CALUMBA ROOT (UNII: V36I2B8LD5) (JATEORHIZA CALUMBA ROOT - UNII:V36I2B8LD5) JATEORHIZA CALUMBA ROOT 4 [hp_X] in 20 mL VIOLA TRICOLOR (UNII: 9Q24RAI43V) (VIOLA TRICOLOR - UNII:9Q24RAI43V) VIOLA TRICOLOR 4 [hp_X] in 20 mL PEUMUS BOLDUS LEAF (UNII: Q4EWM09M3O) (PEUMUS BOLDUS LEAF - UNII:Q4EWM09M3O) PEUMUS BOLDUS LEAF 4 [hp_X] in 20 mL SEMECARPUS ANACARDIUM JUICE (UNII: Y0F0BU8RDU) (SEMECARPUS ANACARDIUM JUICE - UNII:Y0F0BU8RDU) SEMECARPUS ANACARDIUM JUICE 4 [hp_X] in 20 mL ALUMINUM (UNII: CPD4NFA903) (ALUMINUM - UNII:CPD4NFA903) ALUMINUM 12 [hp_X] in 20 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:62106-1132-8 1 in 1 CARTON 02/06/2015 1 20 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 02/06/2015 Labeler - Seroyal USA (018361118) Establishment Name Address ID/FEI Business Operations SAN’UP 401010287 manufacture(62106-1132)