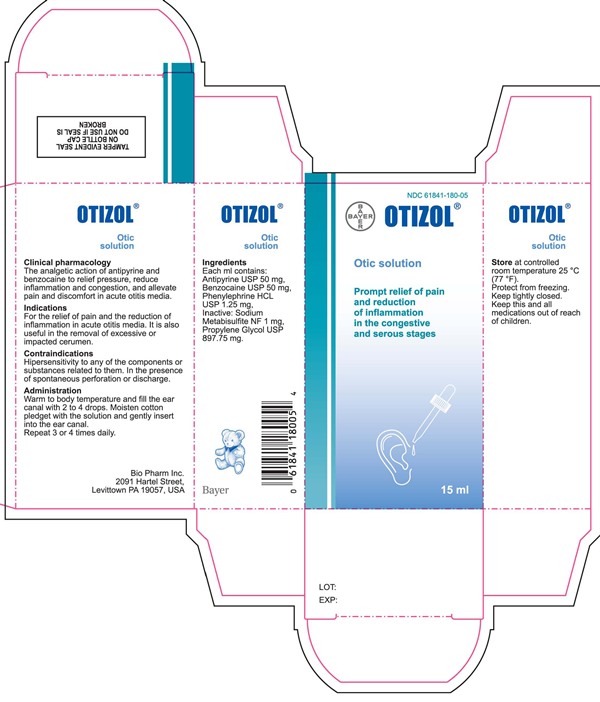
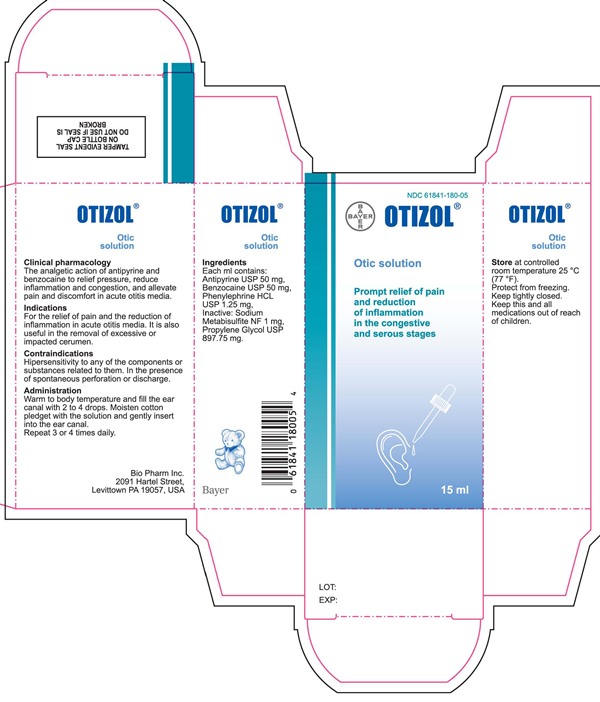
Label: ORTIZOL OTIC SOLUTION- antipyrine, benzocaine, phenylephrine hydrochloride liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 61841-180-05 - Packager: Bayer HealthCare LLC, Consumer Care
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Export only
Drug Label Information
Updated February 5, 2015
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Each ml contains:
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR/PHARMACIST
- WHEN USING
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ORTIZOL OTIC SOLUTION
antipyrine, benzocaine, phenylephrine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61841-180 Route of Administration AURICULAR (OTIC) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ANTIPYRINE (UNII: T3CHA1B51H) (ANTIPYRINE - UNII:T3CHA1B51H) ANTIPYRINE 50 mg in 1 mL BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 50 mg in 1 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 1.25 mg in 1 mL Inactive Ingredients Ingredient Name Strength SODIUM METABISULFITE (UNII: 4VON5FNS3C) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61841-180-05 1 in 1 CARTON 12/19/2014 1 15 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 12/19/2014 Labeler - Bayer HealthCare LLC, Consumer Care (785159372)