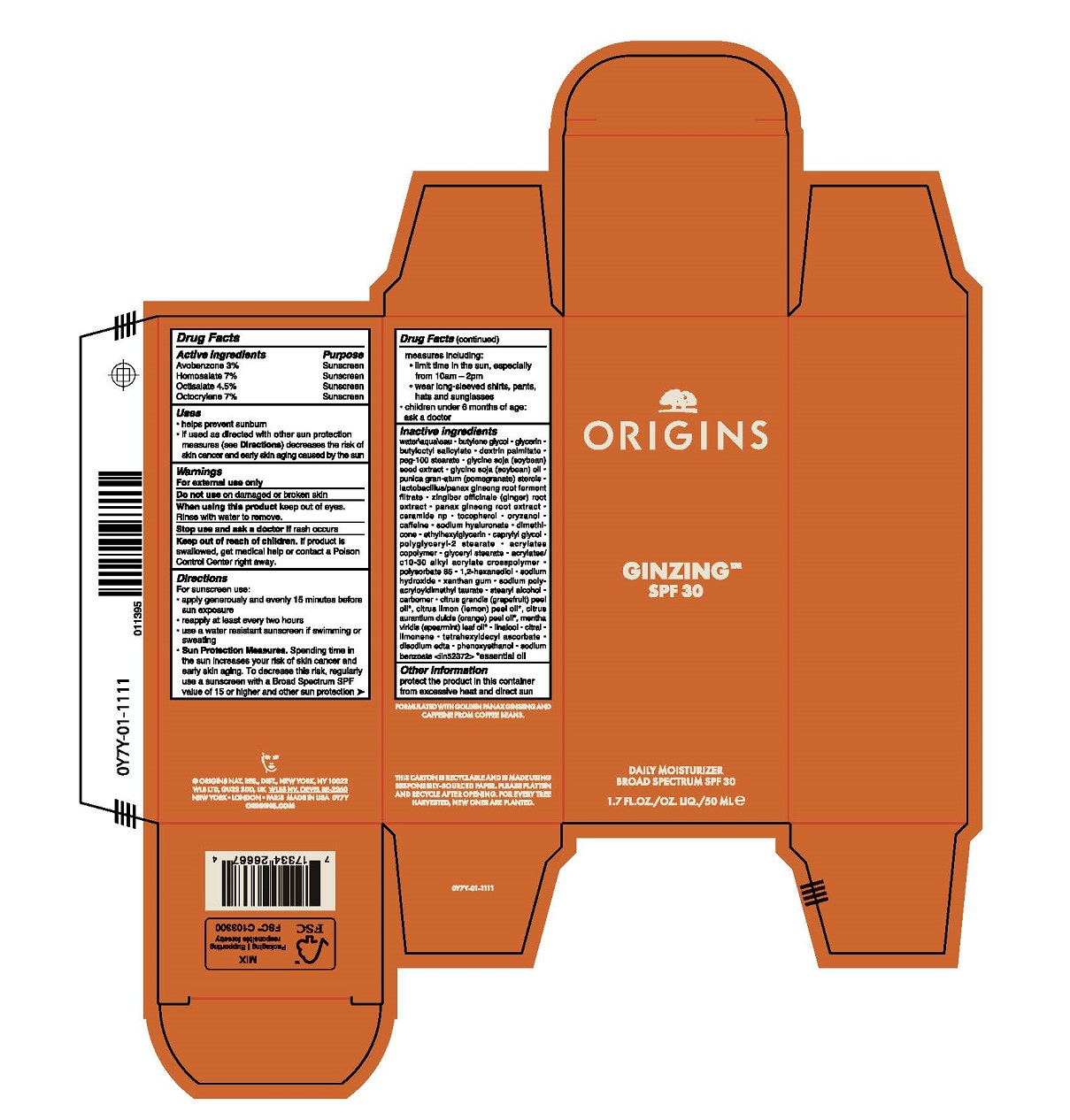
Label: GINZING SPF 30 DAILY MOISTURIZER- avobenzone,homosalate,octisalate,octocrylene cream
- NDC Code(s): 59427-116-01, 59427-116-02, 59427-116-03
- Packager: ORIGINS NATURAL RESOURCES INC.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
For sunscreen use:
- apply generously and evenly 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.–2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive Ingredients
water\aqua\eau,butylene glycol,glycerin,butyloctyl salicylate,dextrin palmitate,peg-100 stearate,glycine soja (soybean) seed extract,glycine soja (soybean) oil,punica granatum (pomegranate) sterols,lactobacillus/panax ginseng root ferment filtrate,zingiber officinale (ginger) root extract,panax ginseng root extract,ceramide np,tocopherol,oryzanol,caffeine,sodium hyaluronate,dimethicone,ethylhexylglycerin,caprylyl glycol,polyglyceryl-2 stearate,acrylates copolymer,glyceryl stearate,acrylates/c10-30 alkyl acrylate crosspolymer,polysorbate 85,1,2-hexanediol,sodium hydroxide,xanthan gum,sodium polyacryloyldimethyl taurate,stearyl alcohol,carbomer,citrus grandis (grapefruit) peel oil*, citrus limon (lemon) peel oil*, citrus aurantium dulcis (orange) peel oil*, mentha viridis (spearmint) leaf oil*,linalool,citral,limonene,tetrahexyldecyl ascorbate,disodium edta,phenoxyethanol,sodium benzoate <iln52372>
- Other Information
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
GINZING SPF 30 DAILY MOISTURIZER
avobenzone,homosalate,octisalate,octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59427-116 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 70 mg in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 70 mg in 1 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 45 mg in 1 mL Inactive Ingredients Ingredient Name Strength GLYCERIN (UNII: PDC6A3C0OX) PEG-100 STEARATE (UNII: YD01N1999R) GINGER (UNII: C5529G5JPQ) ASIAN GINSENG (UNII: CUQ3A77YXI) CERAMIDE NP (UNII: 4370DF050B) TOCOPHEROL (UNII: R0ZB2556P8) HYALURONATE SODIUM (UNII: YSE9PPT4TH) DIMETHICONE (UNII: 92RU3N3Y1O) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) POLYSORBATE 85 (UNII: A7F3N56197) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) SODIUM HYDROXIDE (UNII: 55X04QC32I) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) SPEARMINT OIL (UNII: C3M81465G5) LINALOOL, (+/-)- (UNII: D81QY6I88E) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) PHENOXYETHANOL (UNII: HIE492ZZ3T) SODIUM BENZOATE (UNII: OJ245FE5EU) PUNICA GRANATUM STEROLS (UNII: UKV92KC49T) POLYGLYCERYL-2 STEARATE (UNII: 253MC0P0YV) XANTHAN GUM (UNII: TTV12P4NEE) SODIUM POLYACRYLOYLDIMETHYL TAURATE (UNII: NG5NG5733T) CITRUS MAXIMA FRUIT RIND OIL (UNII: 8U3877WD44) LEMON OIL (UNII: I9GRO824LL) CITRAL (UNII: T7EU0O9VPP) LIMONENE, (+)- (UNII: GFD7C86Q1W) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) DEXTRIN PALMITATE (CORN; 20000 MW) (UNII: 89B2BSF9I3) SOYBEAN (UNII: L7HT8F1ZOD) WATER (UNII: 059QF0KO0R) ORYZANOL (UNII: SST9XCL51M) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ORANGE OIL (UNII: AKN3KSD11B) SOYBEAN OIL (UNII: 241ATL177A) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) CAFFEINE (UNII: 3G6A5W338E) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59427-116-01 1 in 1 CARTON 01/04/2024 1 50 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:59427-116-02 15 mL in 1 TUBE; Type 0: Not a Combination Product 01/04/2024 3 NDC:59427-116-03 5 mL in 1 TUBE; Type 0: Not a Combination Product 01/04/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/04/2024 Labeler - ORIGINS NATURAL RESOURCES INC. (611716283) Registrant - Estee Lauder Companies Inc. (790802086) Establishment Name Address ID/FEI Business Operations The Estee Lauder Inc 802599436 manufacture(59427-116) , pack(59427-116) , label(59427-116)