Label: CHILDRENS LORATADINE- loratadine tablet, chewable
- NDC Code(s): 21130-963-20
- Packager: SAFEWAY
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated March 15, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each chewable tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you haveliver or kidney disease. Your doctor should determine if you need a different dose.
When using this productdo not take more than directed. Taking more than directed may cause drowsiness.
-
Directions
- chew or crush tablets completely before swallowing.
adults and children 6 years and over chew 2 tablets daily; not more than 2 chewable tablets in 24 hours children 2 to under 6 years of age chew 1 tablet daily; not more than 1 chewable tablet in 24 hours children under 2 years of age ask a doctor consumers with liver or kidney disease ask a doctor - Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
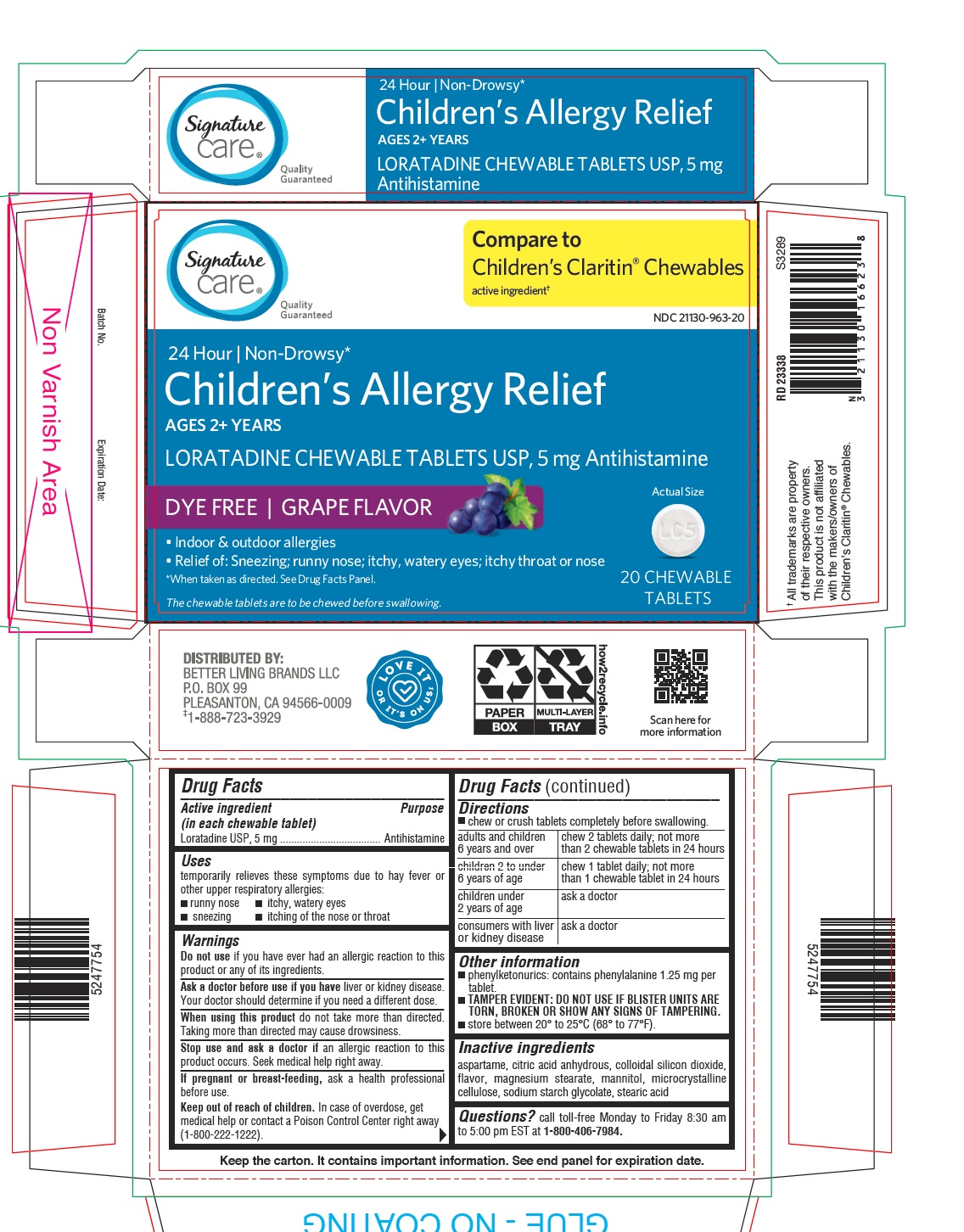
PRINCIPAL DISPLAY PANEL - 5 mg Tablet Blister Pack Carton
Signature
care™
Quality
GuaranteedCompare to
Children's Claritin ®Chewables
active ingredient †NDC 21130-963-20
24 Hour | Non-Drowsy*
Children's Allergy Relief
AGES 2+ YEARS
LORATADINE CHEWABLE TABLETS USP, 5 mg Antihistamine
GRAPE FLAVOR
Actual Size
- Indoor & outdoor allergies
- Relief of: Sneezing; runny nose; itchy, watery eyes; itchy throat or nose
*When taken as directed. See Drug Facts Panel.
The chewable tablets are to be chewed before swallowing.
20 CHEWABLE
TABLETS
-
INGREDIENTS AND APPEARANCE
CHILDRENS LORATADINE
loratadine tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:21130-963 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 5 mg Inactive Ingredients Ingredient Name Strength ASPARTAME (UNII: Z0H242BBR1) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color white (white to off-white) Score no score Shape ROUND Size 10mm Flavor GRAPE Imprint Code LC5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:21130-963-20 2 in 1 CARTON 03/15/2024 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210088 03/15/2024 Labeler - SAFEWAY (009137209) Registrant - OHM LABORATORIES INC. (184769029) Establishment Name Address ID/FEI Business Operations Ohm Laboratories 184769029 manufacture(21130-963)