Label: GLYCERIN, PHENYLEPHRINE HYDROCHLORIDE, PRAMOXINE, WHITE PETROLATUM cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 11673-572-24 - Packager: Target Corporation
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 1, 2010
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
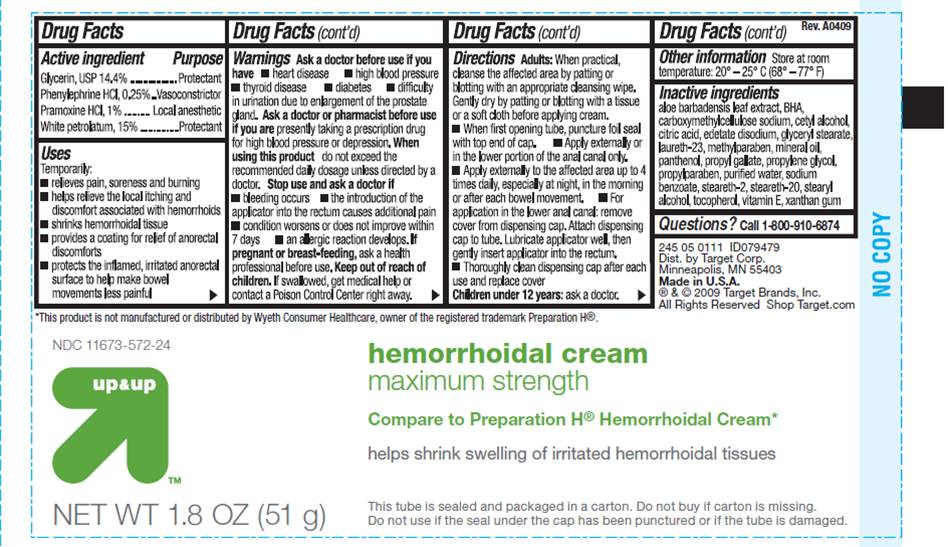
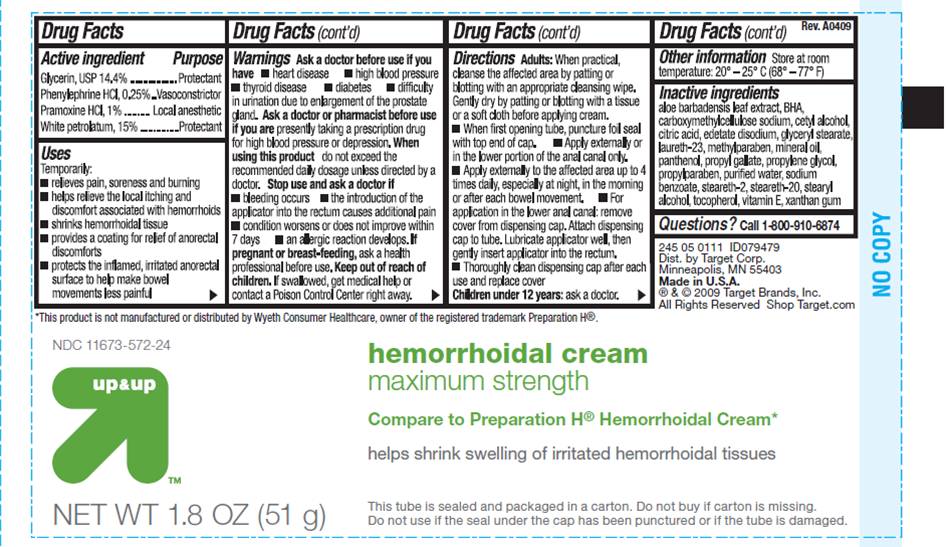
- ACTIVE INGREDIENTS
- PURPOSE
-
USES
- Temporarily relieves pain, soreness and burning
- Helps relieve the local itching and discomfort associated with hemorrhoids
- Temporarily shrinks hemorrhoidal tissue
- Temporarily provides a coating for relief of anorectal discomforts
- Temporarily protects the inflamed, irritated anorectal surface to help make bowel movements less painful
-
WARNINGS
Ask a doctor before use if you have
- heart disease
- thyroid disease
- high blood pressure
- diabetes
- difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
presently taking a prescription drug for high blood pressure or depression.
Stop use and ask a doctor if
- bleeding occurs
- the introduction of the applicator causes additional pain
- condition worsens or does not improve within 7 days
- an allergic reaction develops
- the symptom being treated does not subside or if redness, irritation, swelling, pain or other symptoms develop or increase
-
DIRECTIONS
Adults:
- When practical, cleanse the affected area by patting or blotting with an appropriate cleansing wipe. Gently dry by patting or blotting with a tissue or soft cloth before applying cream.
- When first opening the tube, puncture foil seal with top end of cap
- Apply externally or in the lower portion of the anal canal only
- Apply externally to the affected area up to 4 times daily, especially at night, in the morning or after each bowel movement
- For application in the lower anal canal: remove cover from dispensing cap. Attach dispensing cap to tube. Lubricate applicator well, then gently insert applicator into the rectum.
- Thoroughly clean dispensing cap after each use and replace cover
- SPL UNCLASSIFIED SECTION
- OTHER INFORMATION
-
INACTIVE INGREDIENTS
aloe barbadensis leaf extract, BHA, carboxymethylcellulose sodium, cetyl alcohol, citric acid, edetate disodium, glyceryl stearate, laureth-23, methylparaben, mineral oil, panthenol, propyl gallate, propylene glycol, propylparaben, purified water, sodium benzoate, steareth-2, steareth-20, stearyl alcohol, tocopherol. vitamin E, xanthan gum
- Questions?
-
PACKAGE INFORMATION - TUBE
up and up
NDC 11673-572-24
Hemorrhoidal Cream
maximum strength
Compare to Preparation H® Hemorrhoidal Cream*
Helps shrink swelling of irritated hemorrhoidal tissues
NET WT 1.8 OZ (51 g)
This tube is sealed and packaged in a carton. Do not buy if carton is missing . Do not use if the seal under the cap has been punctured or if the tube is damaged.
*This product is not manufactured or distributed by Wyeth Consumer Healthcare, owner of the registered trademark Preparation H®.
Dist. by Target Corp.
Minneapolis, MN 55403
Made in U.S.A.
® and © 2009 Target Brands, Inc.
All Rights Reserved
Shop Target.com
-
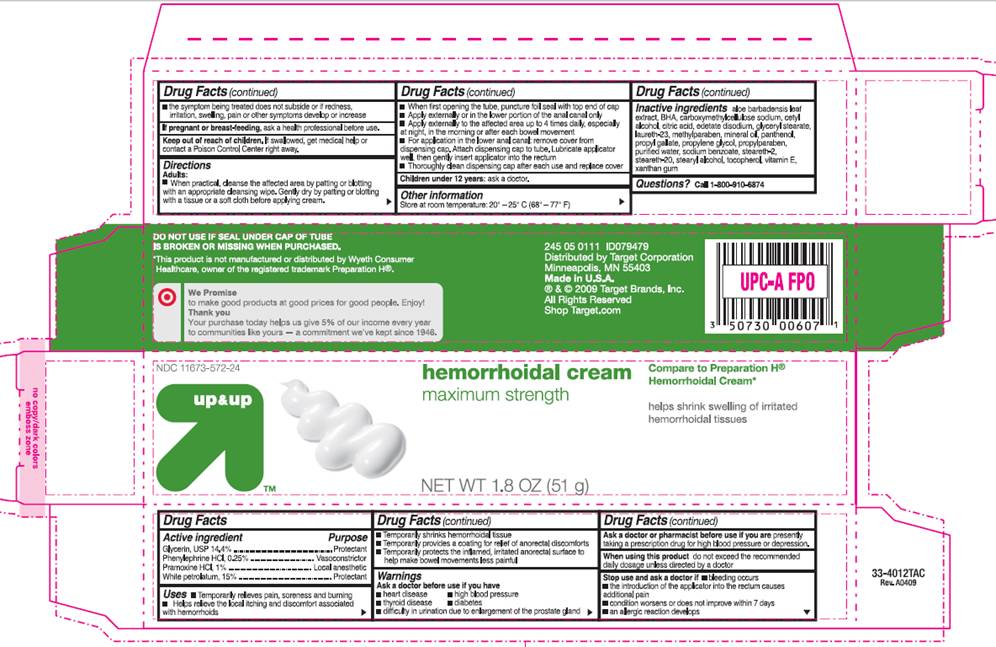
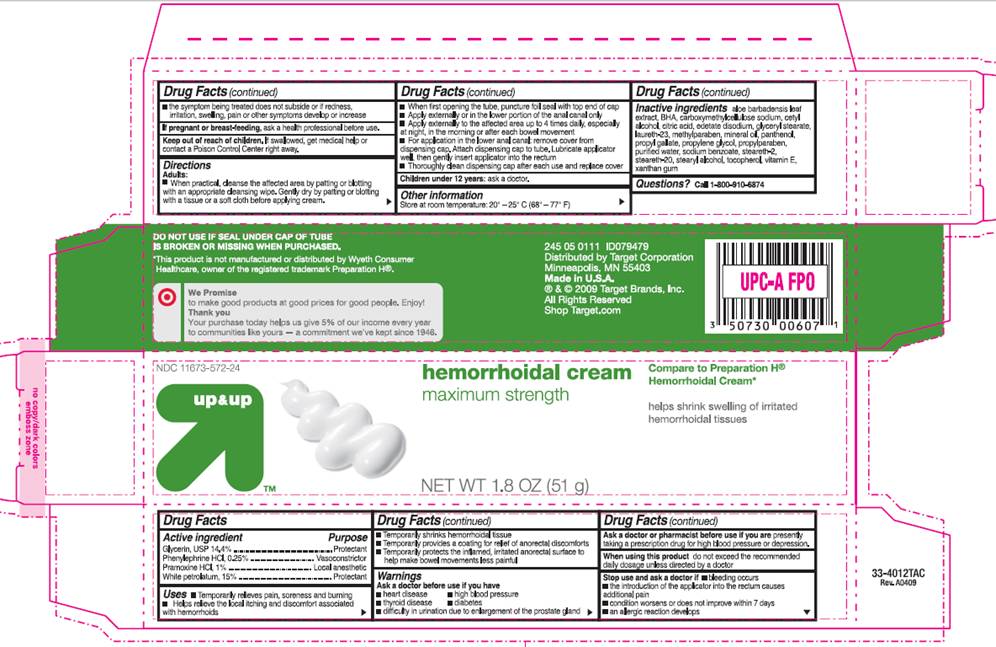
PACKAGE INFORMATION - BOX
up and up
NDC 11673-572-24
Hemorrhoidal Cream
maximum strength
Compare to Preparation H® Hemorrhoidal Cream*
Helps shrink swelling of irritated hemorrhoidal tissues
NET WT 1.8 OZ (51 g)
DO NOT USE IF SEAL UNDER CAP OF TUBE IS BROKEN OR MISSING WHEN PURCHASED.
*This product is not manufactured or distributed by Wyeth Consumer Healthcare, owner of the registered trademark Preparation H®.
Distributed by Target Corporation
Minneapolis, MN 55403
Made in U.S.A.
® and © 2009 Target Brands, Inc.
All Rights Reserved
Shop Target.com
-
INGREDIENTS AND APPEARANCE
GLYCERIN, PHENYLEPHRINE HYDROCHLORIDE, PRAMOXINE, WHITE PETROLATUM
glycerin, phenylephrine hydrochloride, pramoxine, white petrolatum creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11673-572 Route of Administration RECTAL, TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCERIN (UNII: PDC6A3C0OX) (GLYCERIN - UNII:PDC6A3C0OX) GLYCERIN 0.144 mg in 1 g PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 0.0025 mg in 1 g PRAMOXINE HYDROCHLORIDE (UNII: 88AYB867L5) (PRAMOXINE - UNII:068X84E056) PRAMOXINE HYDROCHLORIDE 0.01 mg in 1 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 0.15 mg in 1 g Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) CETYL ALCOHOL (UNII: 936JST6JCN) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LAURETH-23 (UNII: N72LMW566G) METHYLPARABEN (UNII: A2I8C7HI9T) LIGHT MINERAL OIL (UNII: N6K5787QVP) PANTHENOL (UNII: WV9CM0O67Z) PROPYL GALLATE (UNII: 8D4SNN7V92) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) ALPHA-TOCOPHEROL (UNII: H4N855PNZ1) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11673-572-24 1 in 1 BOX 1 51 g in 1 TUBE, WITH APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part346 04/01/2009 Labeler - Target Corporation (006961700)