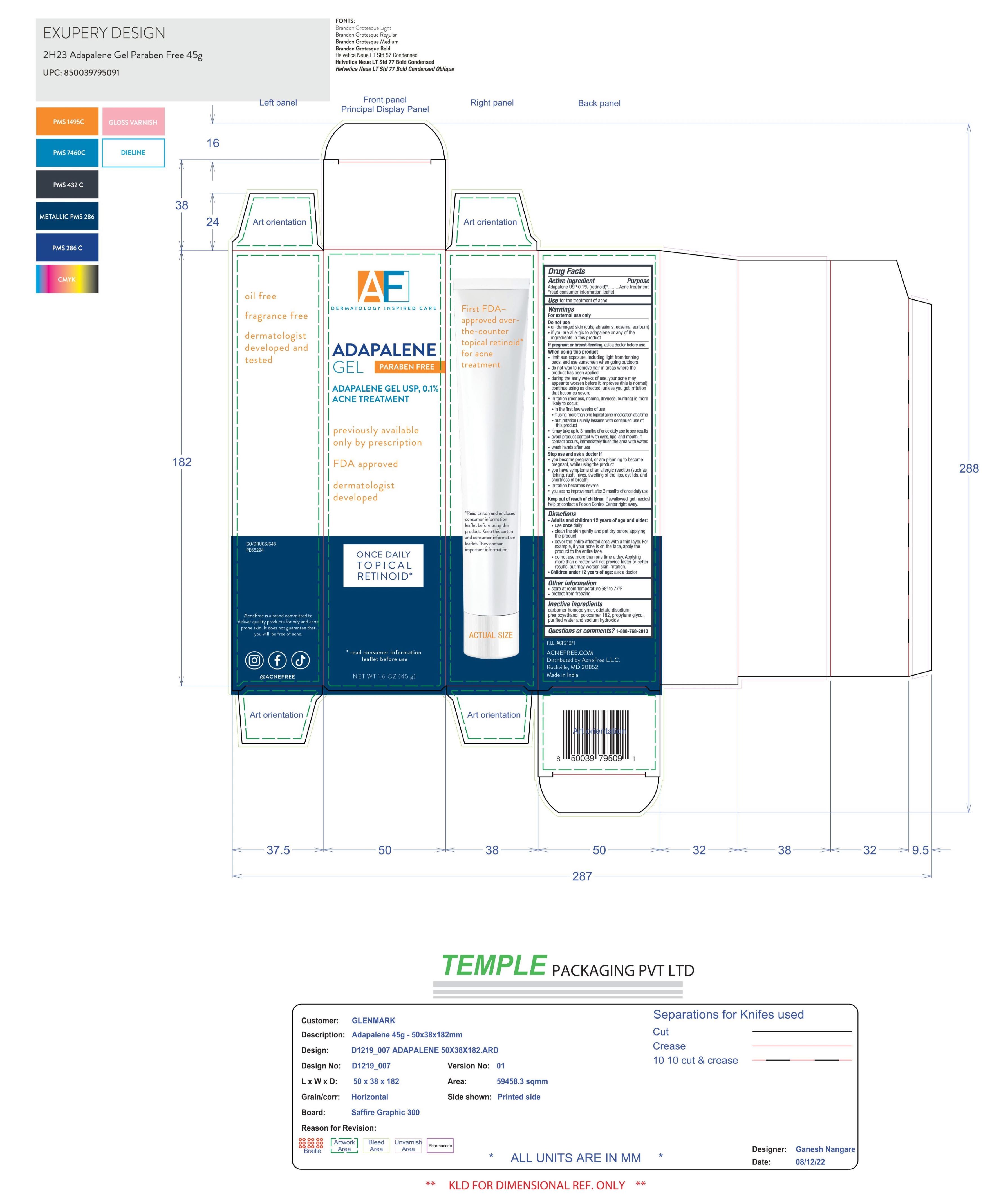
Label: ADAPALENE- adaplane 0.1% gel
- NDC Code(s): 80861-115-01
- Packager: Acne Free, LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated June 19, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- Use
-
Warnings
For external use only
• on damaged skin (cuts, abrasions, eczema, sunburn)
• if you are allergic to adapalene or any of the ingredients in this product
When using this product
When using this product • • • • • • • limit sun exposure, including light from tanning beds, and use sunscreen when going outdoors do not wax to remove hair in areas where the product has been applied during the early weeks of use, your acne may appear to worsen before it improves (this is normal); continue using as directed, unless you get irritation that becomes severe irritation (redness, itching, dryness, burning) is more likely to occur: • in the first few weeks of use • if using more than one topical acne medication at a time • but irritation usually lessens with continued use of this product it may take up to 3 months of once daily use to see results avoid product contact with eyes, lips, and mouth. If contact occurs, immediately flush the area with water. wash hands after use
Stop use and ask doctor if
you become pregnant, or are planning to become pregnant, while using the product you have symptoms of an allergic reaction (such as itching, rash, hives, swelling of the lips, eyelids, and shortness of breath) irritation becomes severe you see no improvement after 3 months of once daily use
-
Directions
Adults and children 12 years of age and older:
use once daily clean the skin gently and pat dry before applying the product cover the entire affected area with a thin layer. For example, if your acne is on the face, apply the product to the entire face. do not use more than one time a day. Applying more than directed will not provide faster or better results, but may worsen skin irritation. • Adults and children 12 years of age and older: • Children under 12 years of age: ask a doctor
- Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ADAPALENE
adaplane 0.1% gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80861-115 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADAPALENE (UNII: 1L4806J2QF) (ADAPALENE - UNII:1L4806J2QF) ADAPALENE 0.1 g in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE C (UNII: 4Q93RCW27E) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80861-115-01 1 g in 1 TUBE; Type 0: Not a Combination Product 01/05/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091314 01/05/2024 Labeler - Acne Free, LLC (122237140) Establishment Name Address ID/FEI Business Operations Glenmark Pharmaceuticals 677318665 manufacture(80861-115)