Label: ALERTNESS AID- caffeine tablet
- NDC Code(s): 68016-680-16, 68016-680-40
- Packager: Chain Drug Consortium
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated May 10, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each tablet)
- Purpose
- Use
-
Warnings
For occasional use only
Caffeine warning: The recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages while taking this product because too much caffeine may cause nervousness, irritability, sleeplessness, and, occasionally, rapid heart beat.
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
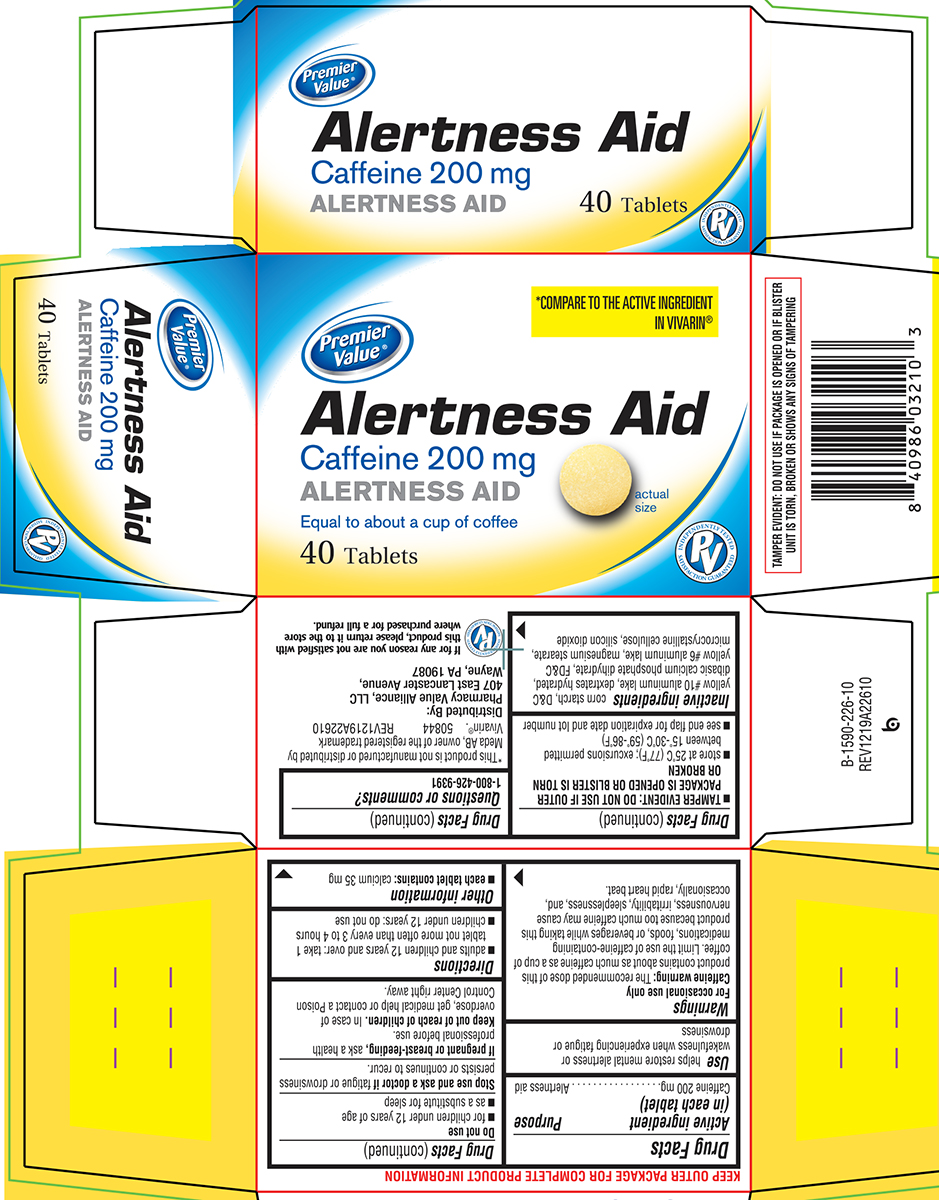
Principal Display Panel
Premier
Value®*COMPARE TO THE ACTIVE INGREDIENT
IN VIVARIN®Alertness Aid
Caffeine 200 mg
ALERTNESS AIDEqual to about a cup of coffee
40 Tablets
actual
sizeINDEPENDENTLY TESTED
PV
SATISFACTION GUARANTEEDTAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING*This product is not manufactured or distributed by
Meda AB, owner of the registered trademark
Vivarin®. 50844 REV1219A22610Distributed By:
Pharmacy Value Alliance, LLC
407 East Lancaster Avenue,
Wayne, PA 19087If for any reason you are not satisfied with
this product, please return it to the store
where purchased for a full refund.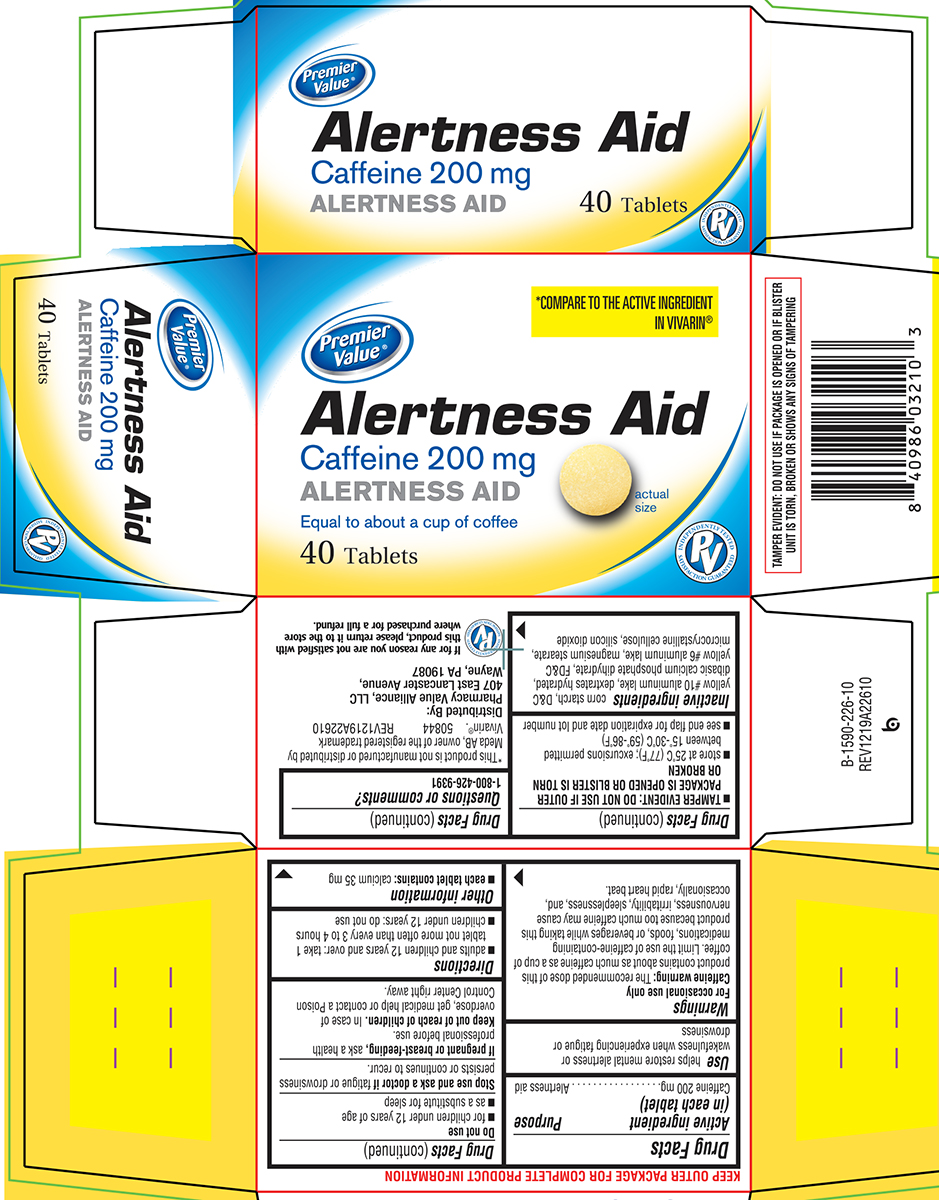
44-226
-
INGREDIENTS AND APPEARANCE
ALERTNESS AID
caffeine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68016-680 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 200 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 ALUMINUM LAKE (UNII: CQ3XH3DET6) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C YELLOW NO. 6 ALUMINUM LAKE (UNII: GYP6Z2JR6Q) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) Product Characteristics Color yellow Score no score Shape ROUND Size 11mm Flavor Imprint Code 44;226 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68016-680-40 5 in 1 CARTON 11/21/1996 1 8 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC:68016-680-16 2 in 1 CARTON 11/21/1996 05/17/2023 2 8 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M011 11/21/1996 Labeler - Chain Drug Consortium (101668460) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 pack(68016-680) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 manufacture(68016-680) , pack(68016-680) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 pack(68016-680) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 117025878 manufacture(68016-680)