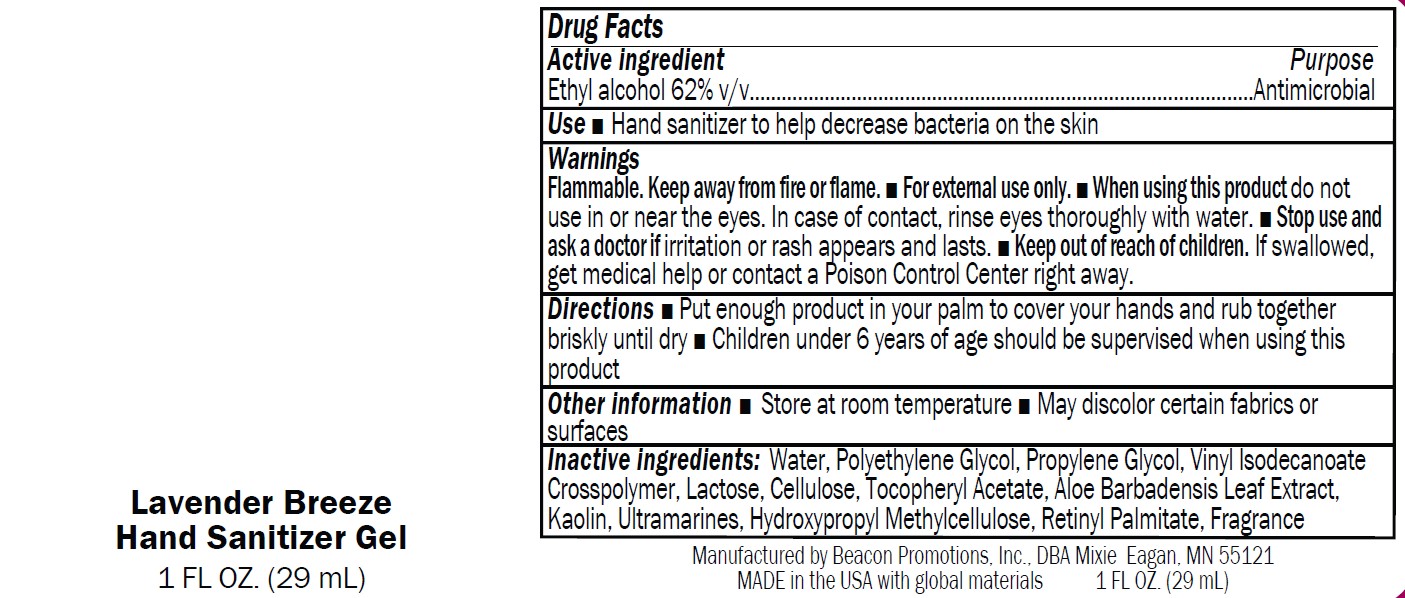
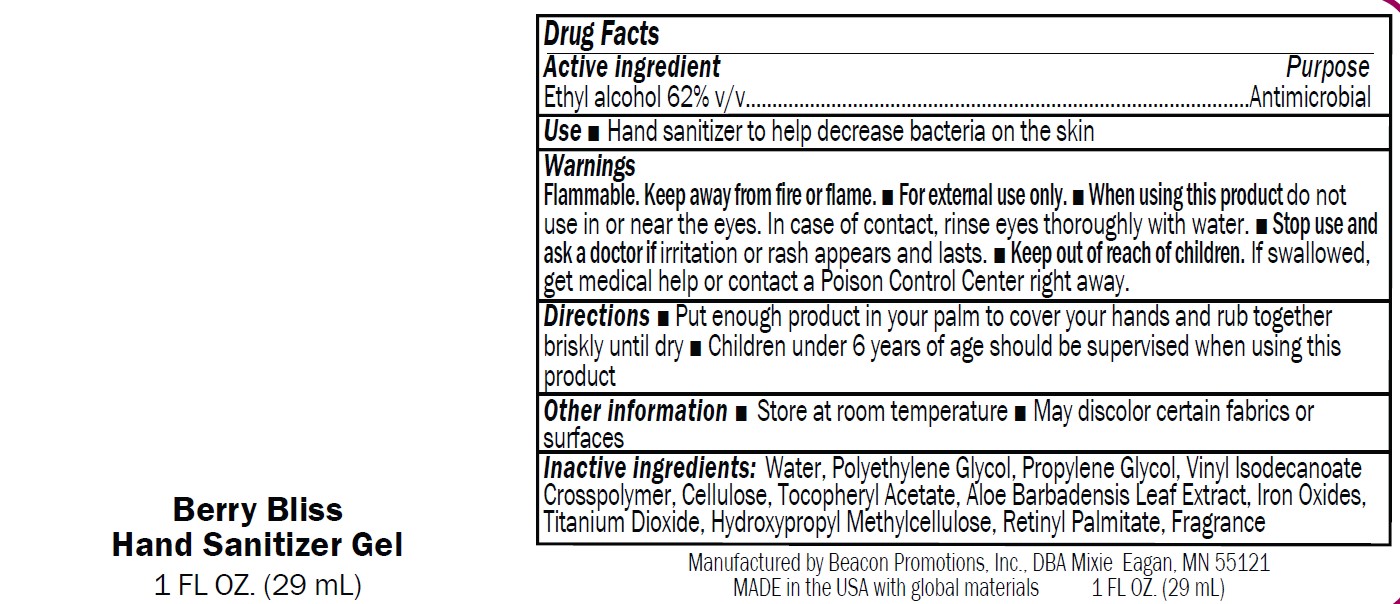
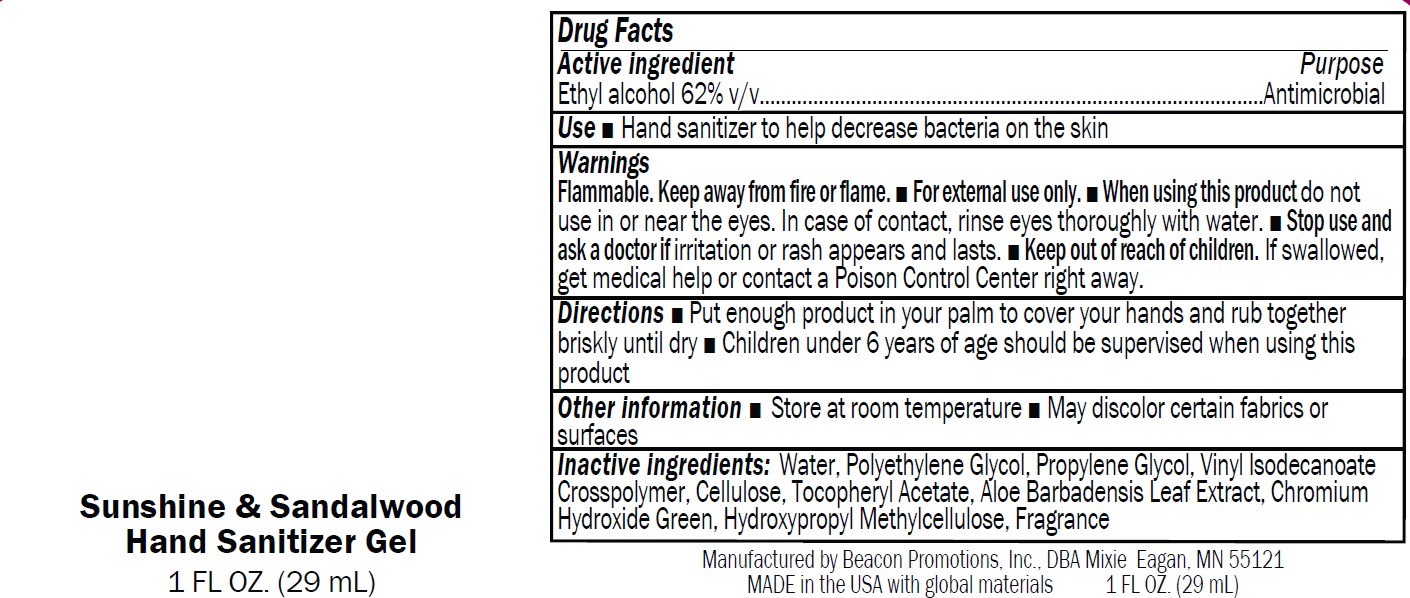
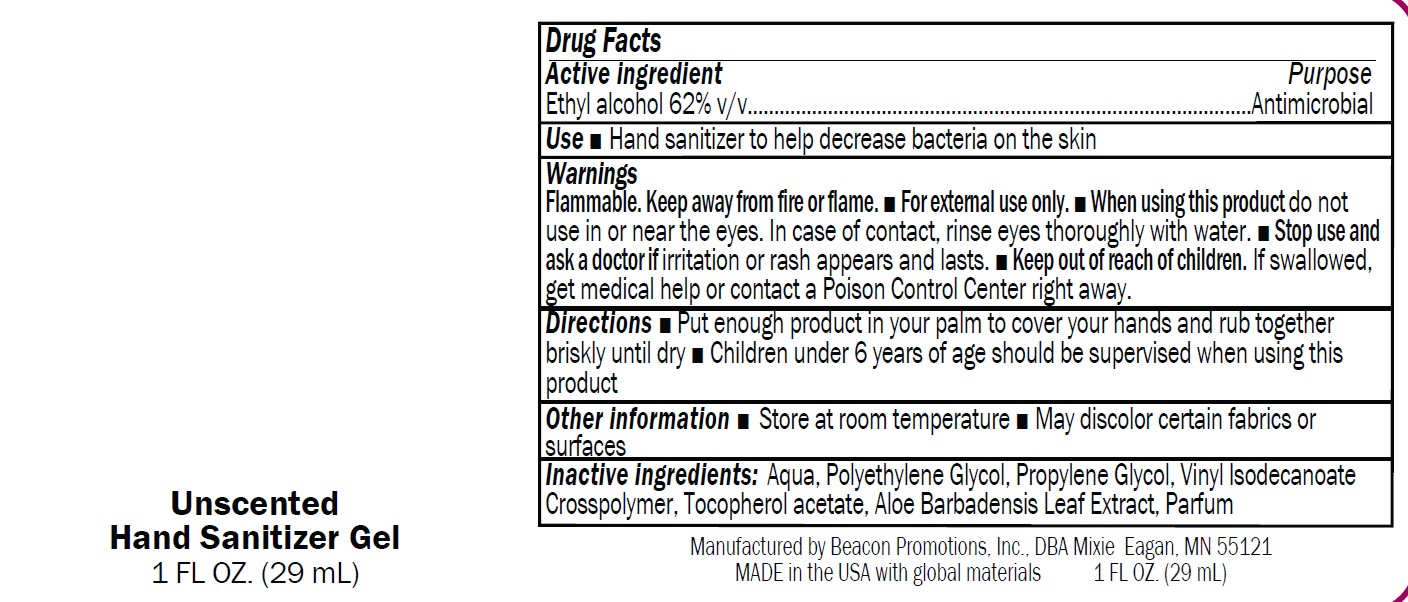
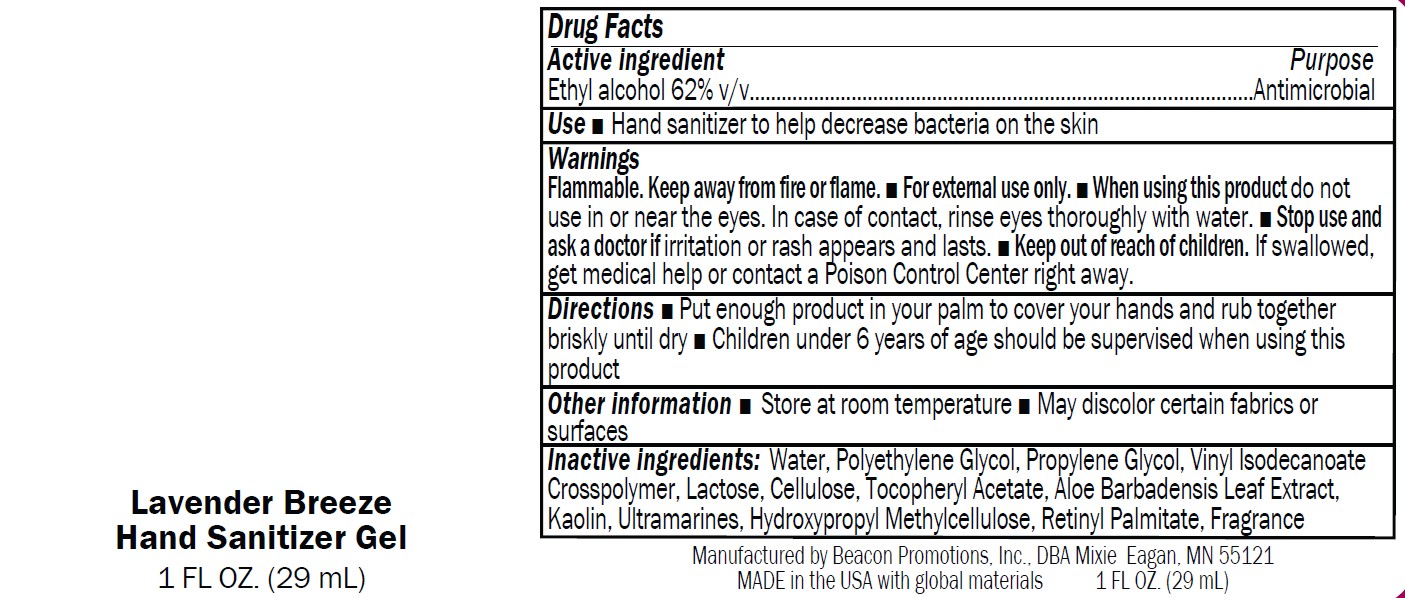
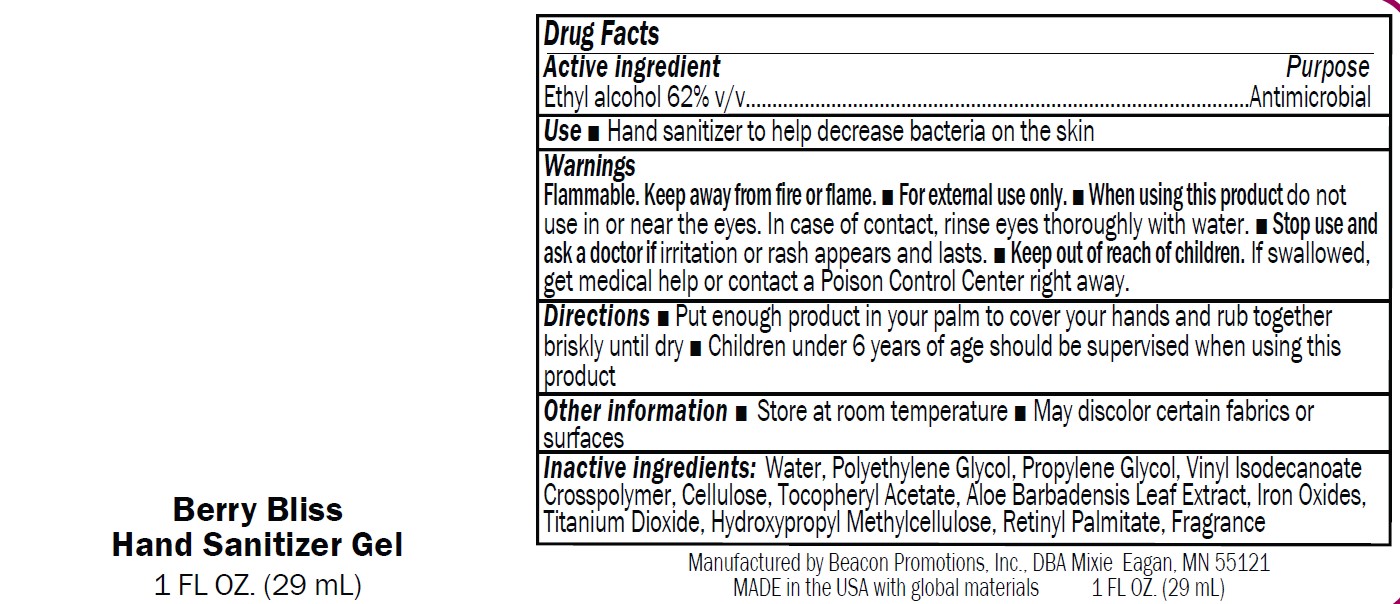
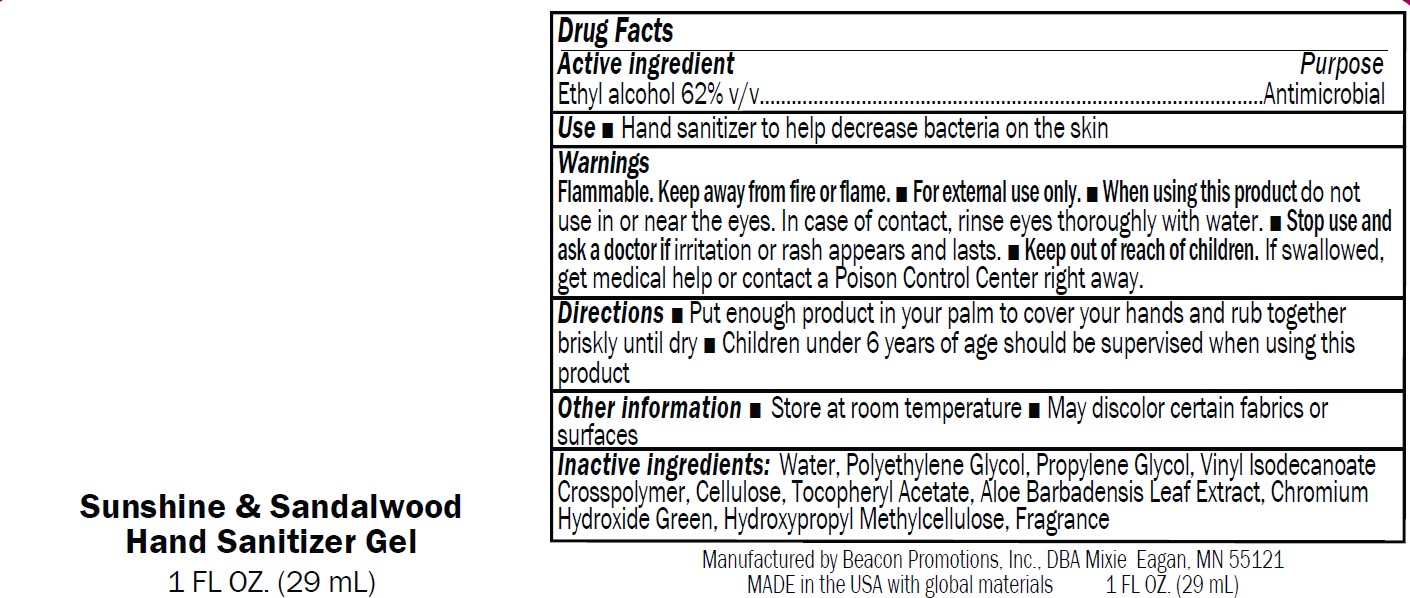
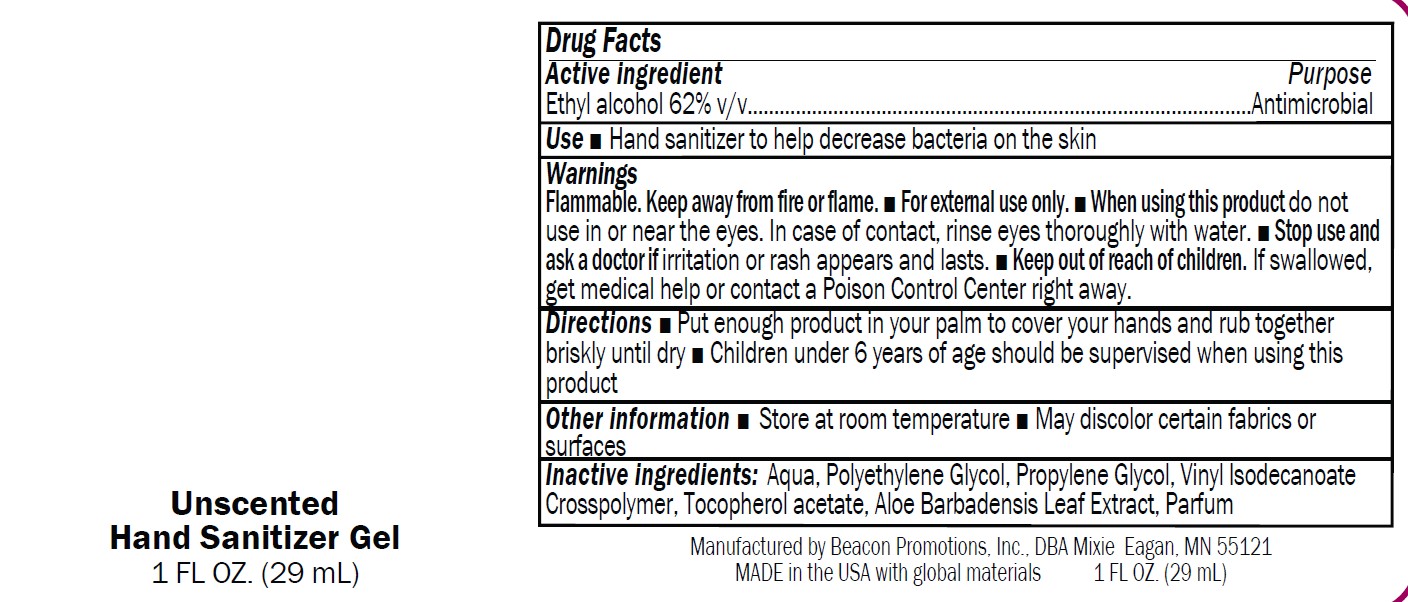
Label: SANMO-WH- antibacterial hand sanitizer with white beads gel
SANMO-RD- hand sanitizer with red beads gel
SANMO-BL- antibacterial hand sanitizer with blue beads gel
SANMO-GR- antibacterial hand sanitizer with green beads gel
SAN-UN- antibacterial hand sanitizer unscented gel
-
NDC Code(s):
70445-600-01,
70445-600-02,
70445-600-03,
70445-600-04, view more70445-600-05, 70445-600-06, 70445-600-07, 70445-600-08, 70445-600-09, 70445-601-01, 70445-601-02, 70445-601-03, 70445-601-04, 70445-601-05, 70445-601-06, 70445-601-07, 70445-601-08, 70445-601-09, 70445-602-01, 70445-602-02, 70445-602-03, 70445-602-04, 70445-602-05, 70445-602-06, 70445-602-07, 70445-602-08, 70445-602-09, 70445-603-01, 70445-603-02, 70445-603-03, 70445-603-04, 70445-603-05, 70445-603-06, 70445-603-07, 70445-603-08, 70445-603-09, 70445-604-01, 70445-604-02, 70445-604-03, 70445-604-04, 70445-604-05, 70445-604-06, 70445-604-07, 70445-604-08, 70445-604-09
- Packager: Beacon Promotions, Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug facts
- USE
-
Warnings
Flammable. Keep away from fire or flame.
■ For external use only.
■ When using this product do not
use in or near the eyes. In case of contact, rinse eyes thoroughly with water.■ Stop use and
ask a doctor if irritation or rash appears and lasts.■ Keep out of reach of children.
If swallowed,get medical help or contact a Poison Control Center right away
- Directions
- Drug Facts
- Inactive Ingredients
- Other
- SANMO Blue Beads
- SANMO Red Beads
- SANMO Green Beads
- SANMO - Unscented
- SANMO White Beads
-
INGREDIENTS AND APPEARANCE
SANMO-WH
antibacterial hand sanitizer with white beads gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70445-601 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 63 mL in 100 mL Inactive Ingredients Ingredient Name Strength ACRYLATES/VINYL ISODECANOATE CROSSPOLYMER (10000 MPA.S NEUTRALIZED AT 0.5%) (UNII: 2N8MDB79NA) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) ALOE (UNII: V5VD430YW9) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70445-601-01 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 2 NDC:70445-601-02 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 3 NDC:70445-601-03 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 4 NDC:70445-601-04 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 5 NDC:70445-601-05 240 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 6 NDC:70445-601-06 240 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/03/2024 7 NDC:70445-601-07 300 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/03/2024 8 NDC:70445-601-08 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 9 NDC:70445-601-09 480 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/03/2024 SANMO-RD
hand sanitizer with red beads gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70445-600 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ACRYLATES/VINYL ISODECANOATE CROSSPOLYMER (10000 MPA.S NEUTRALIZED AT 0.5%) (UNII: 2N8MDB79NA) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) FERRIC OXIDE RED (UNII: 1K09F3G675) ALOE (UNII: V5VD430YW9) POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70445-600-01 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 2 NDC:70445-600-02 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 3 NDC:70445-600-03 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 4 NDC:70445-600-04 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 5 NDC:70445-600-05 240 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 6 NDC:70445-600-06 240 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/01/2024 7 NDC:70445-600-07 300 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/01/2024 8 NDC:70445-600-08 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/01/2024 9 NDC:70445-600-09 480 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/01/2024 SANMO-BL
antibacterial hand sanitizer with blue beads gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70445-602 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 63 mL in 100 mL Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) WATER (UNII: 059QF0KO0R) ACRYLATES/VINYL ISODECANOATE CROSSPOLYMER (10000 MPA.S NEUTRALIZED AT 0.5%) (UNII: 2N8MDB79NA) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) KAOLIN (UNII: 24H4NWX5CO) ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) ALOE (UNII: V5VD430YW9) ULTRAMARINE BLUE (UNII: I39WR998BI) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70445-602-01 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 2 NDC:70445-602-02 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 3 NDC:70445-602-03 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 4 NDC:70445-602-04 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 5 NDC:70445-602-05 240 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 6 NDC:70445-602-06 240 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/03/2024 7 NDC:70445-602-07 300 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/03/2024 8 NDC:70445-602-08 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 9 NDC:70445-602-09 480 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/03/2024 SANMO-GR
antibacterial hand sanitizer with green beads gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70445-603 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE (UNII: V5VD430YW9) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) WATER (UNII: 059QF0KO0R) ACRYLATES/VINYL ISODECANOATE CROSSPOLYMER (10000 MPA.S NEUTRALIZED AT 0.5%) (UNII: 2N8MDB79NA) MICROCRYSTALLINE CELLULOSE 101 (UNII: 7T9FYH5QMK) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) CHROMIUM HYDROXIDE GREEN (UNII: RV8FT8XF5R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70445-603-01 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 2 NDC:70445-603-02 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 3 NDC:70445-603-03 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 4 NDC:70445-603-04 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 5 NDC:70445-603-05 240 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 6 NDC:70445-603-06 240 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/03/2024 7 NDC:70445-603-07 300 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/03/2024 8 NDC:70445-603-08 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 9 NDC:70445-603-09 480 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/03/2024 SAN-UN
antibacterial hand sanitizer unscented gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70445-604 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 63 mL in 100 mL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALOE (UNII: V5VD430YW9) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70445-604-01 15 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 2 NDC:70445-604-02 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 3 NDC:70445-604-03 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 4 NDC:70445-604-04 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 5 NDC:70445-604-05 240 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 6 NDC:70445-604-06 240 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/03/2024 7 NDC:70445-604-07 300 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/03/2024 8 NDC:70445-604-08 480 mL in 1 BOTTLE; Type 0: Not a Combination Product 01/03/2024 9 NDC:70445-604-09 480 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/03/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/03/2024 Labeler - Beacon Promotions, Inc. (119056382) Establishment Name Address ID/FEI Business Operations Beacon Promotions inc. 119056382 manufacture(70445-601, 70445-602, 70445-603, 70445-604, 70445-600)