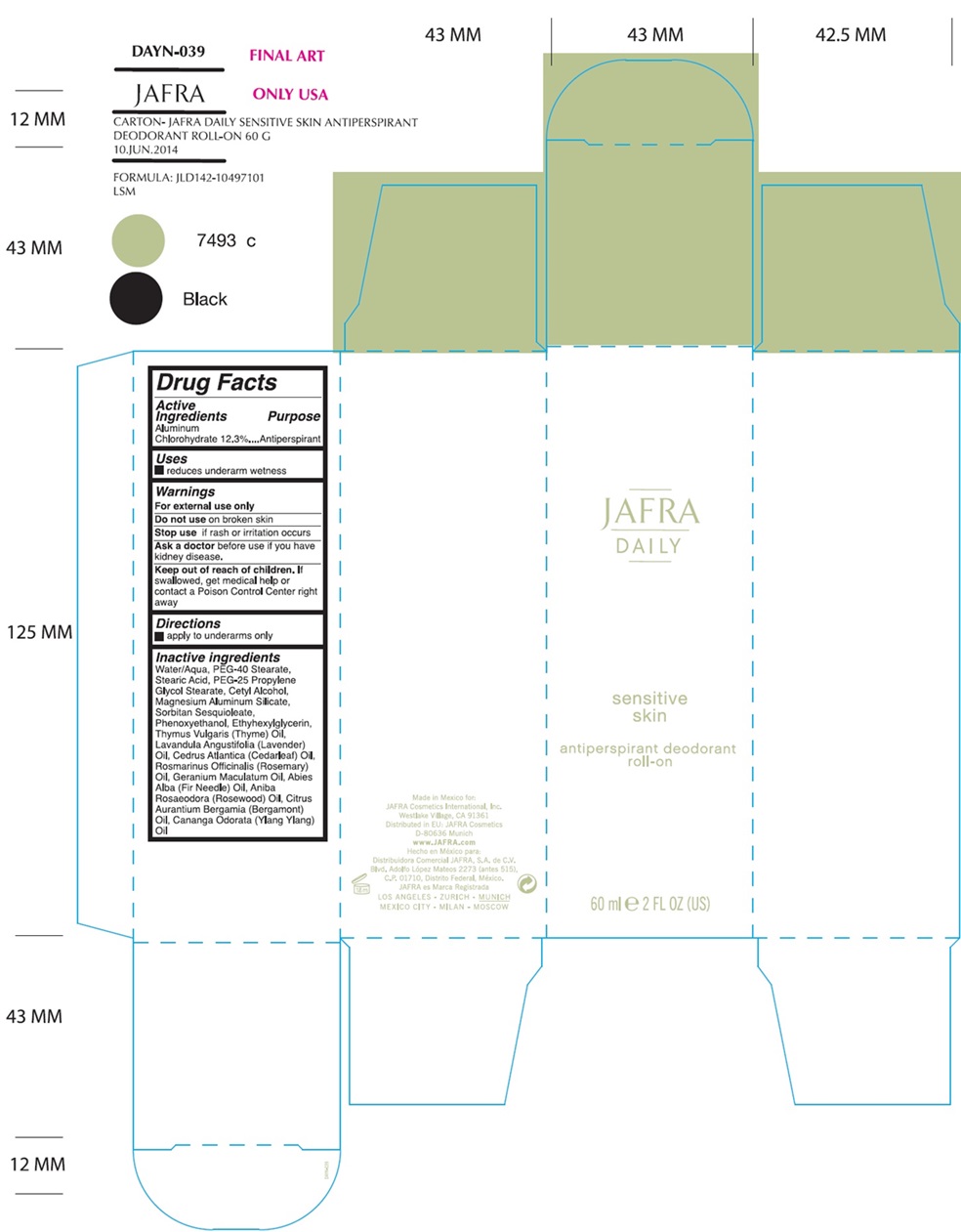
Label: JAFRA DAILY SENSITIVE SKIN ANTIPERSPIRANT DEODORANT ROLL-ON- aluminum chlorohydrate liquid
- NDC Code(s): 68828-705-01, 68828-705-02
- Packager: Jafra Cosmetics International
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
- Directions
-
INACTIVE INGREDIENT
Inactive Ingredients
Water/Aqua, PEG-40 Stearate, Stearic Acid, PEG-25 Propylene Glycol Stearate, Cetyl Alcohol, Magnesium Aluminum Silicate, Sorbitan Sesquioleate, Phenoxyethanol, Ethylhexylglycerin, Thymus Vulgaris (Thyme) Oil, Lavandula Angustifolia (Lavender) Oil, Cedrus Atlantica (Cedarleaf) Oil, Rosmarinus Officinalis (Rosemary) Oil, Geranium Maculatum Oil, Abies Alba (Fir Needle) Oil, Aniba Roseadora (Rosewood) Oil, Citrus Aurantium Bergamia (Bergamot) Oil, Cananga Odorata (Ylang Ylang) Oil - Product label
-
INGREDIENTS AND APPEARANCE
JAFRA DAILY SENSITIVE SKIN ANTIPERSPIRANT DEODORANT ROLL-ON
aluminum chlorohydrate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-705 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM CHLOROHYDRATE (UNII: HPN8MZW13M) (ALUMINUM CHLOROHYDRATE - UNII:HPN8MZW13M) ALUMINUM CHLOROHYDRATE 12.3 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PEG-40 STEARATE (UNII: ECU18C66Q7) PEG-25 PROPYLENE GLYCOL STEARATE (UNII: X21KPH4633) STEARIC ACID (UNII: 4ELV7Z65AP) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) CETYL ALCOHOL (UNII: 936JST6JCN) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) THYME OIL (UNII: 2UK410MY6B) LAVENDER OIL (UNII: ZBP1YXW0H8) CEDAR LEAF OIL (UNII: BJ169U4NLG) ROSEMARY OIL (UNII: 8LGU7VM393) GERANIUM MACULATUM ROOT OIL (UNII: H2E371EDYX) ABIES ALBA LEAF OIL (UNII: G49QS877DA) ROSEWOOD OIL (UNII: F2522O5L7B) BERGAMOT OIL (UNII: 39W1PKE3JI) YLANG-YLANG OIL (UNII: 8YOY78GNNX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-705-02 1 in 1 CARTON 12/31/2021 1 NDC:68828-705-01 60 mL in 1 BOTTLE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M019 12/31/2021 Labeler - Jafra Cosmetics International (041676479) Registrant - Jafra Cosmetics International (041676479) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-705)