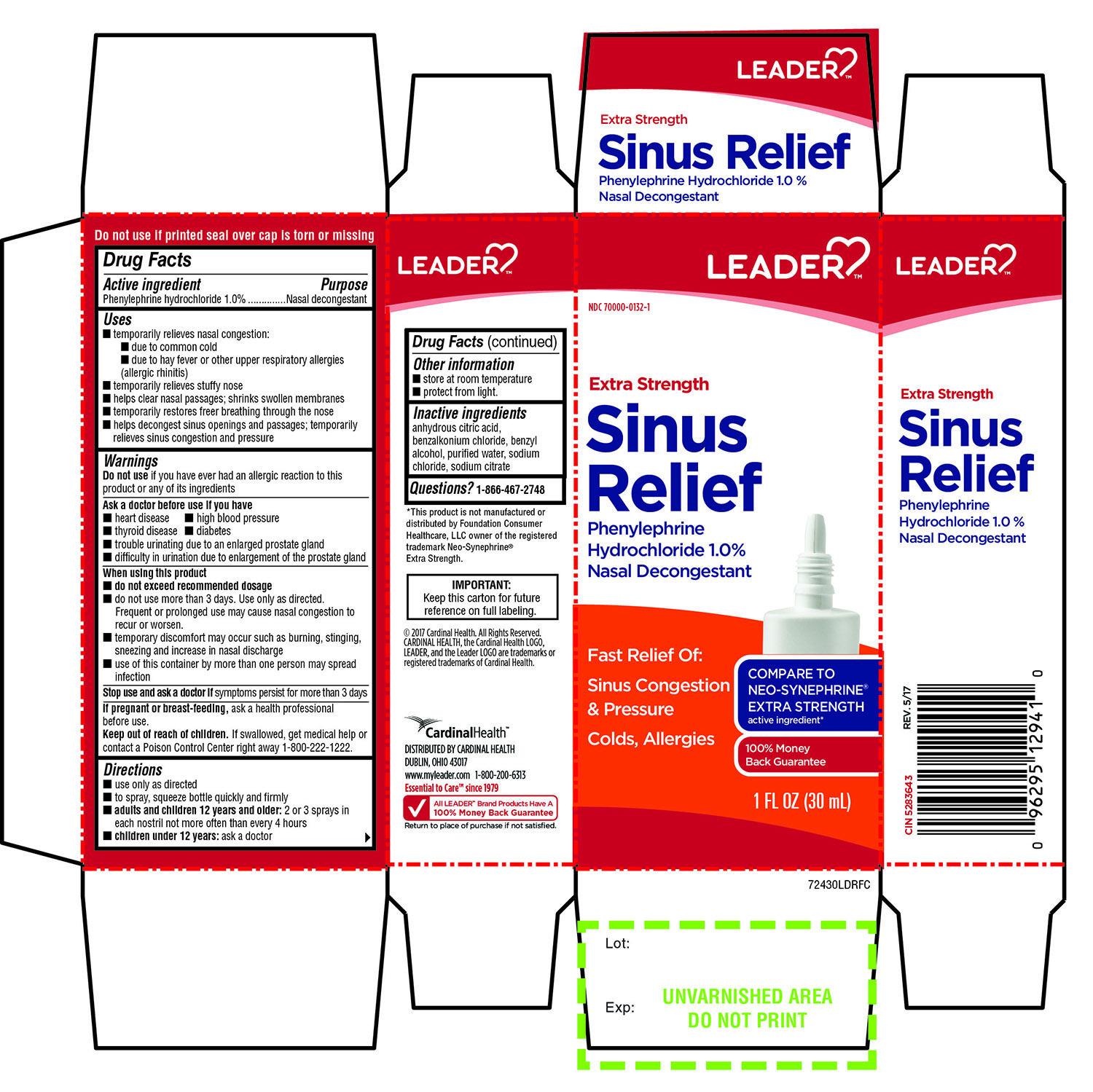
Label: EXTRA STRENGTH SINUS RELIEF NASAL DECONGESTANT- phenylephrine hydrochloride spray
- NDC Code(s): 70000-0132-1
- Packager: CARDINAL HEALTH
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: OTC monograph final
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 4, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredients
- Purpose
-
Uses
- ▪
- temporarily relieves nasal congestion:
- ▪
- due to common cold
- ▪
- due to hay fever or other upper respiratory allergies (allergic rhinitis)
- ▪
- temporarily relieves stuffy nose.
- ▪
- helps clear nasal passages; shrinks swollen membranes
- ▪
- temporarily restores freer breathing through the nose
- ▪
- helps decongest sinus openings and passages; temporarily relieves sinus congestion and pressure
- Warnings
- Ask a doctor before use if you have
-
When using this product
- ▪
- do not exceed recommended dosage
- ▪
- do not use for more than 3 days. Use only as directed.
Frequent or prolonged use may cause nasal congestion to recur or worsen
- ▪
- temporary discomfort may occur such as burning, stinging, sneezing and increase in nasal discharge
- ▪
- use of this container by more than one person may spread infection
- Stop use and ask a doctor if
- If pregnant or breast-feeding
- Keep out of reach of children.
- Directions
- Other information
- Inactive ingredients
-
Principal Display
LEADER
NDC 70000-0132-1
COMPARE TO NEO-SYNEPHRINE® EXTRA STRENGTH active ingredient
Extra Strength
Sinus Relief
Phenylephrine hydrochloride 1.0 %
Nasal Decongestant
Fast Relief Of:
Sinus Congestion & Pressure
- Colds, Allergies
1 FL OZ (30 mL)
* This product is not manufactured or distributed by Foundation Consumer Healthcare LLC owner of the registered trademark Neo-Synephrine® Extra Strength.
IMPORTANT: Keep this carton for future reference on full labeling.
Do not use if printed seal over cap is torn or missing
©2017 Cardinal Health, All Rights Reserved , CARDINAL HEALTH ,the Cardinal Health LOGO, LEADER, and the Leader LOGO are trademarks or registered trademarks of Cardinal Health.
Cardinal Health™
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
1-800-200-6313
Essential to care™ since 1979
* This product is not manufactured or distributed by Foundation Consumer Healthcare LLC owner of the registered trademark Neo-Synephrine® Extra Strength.
-
INGREDIENTS AND APPEARANCE
EXTRA STRENGTH SINUS RELIEF NASAL DECONGESTANT
phenylephrine hydrochloride sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70000-0132 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) BENZYL ALCOHOL (UNII: LKG8494WBH) WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70000-0132-1 1 in 1 CARTON 06/06/2017 1 30 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/06/2017 Labeler - CARDINAL HEALTH (063997360)