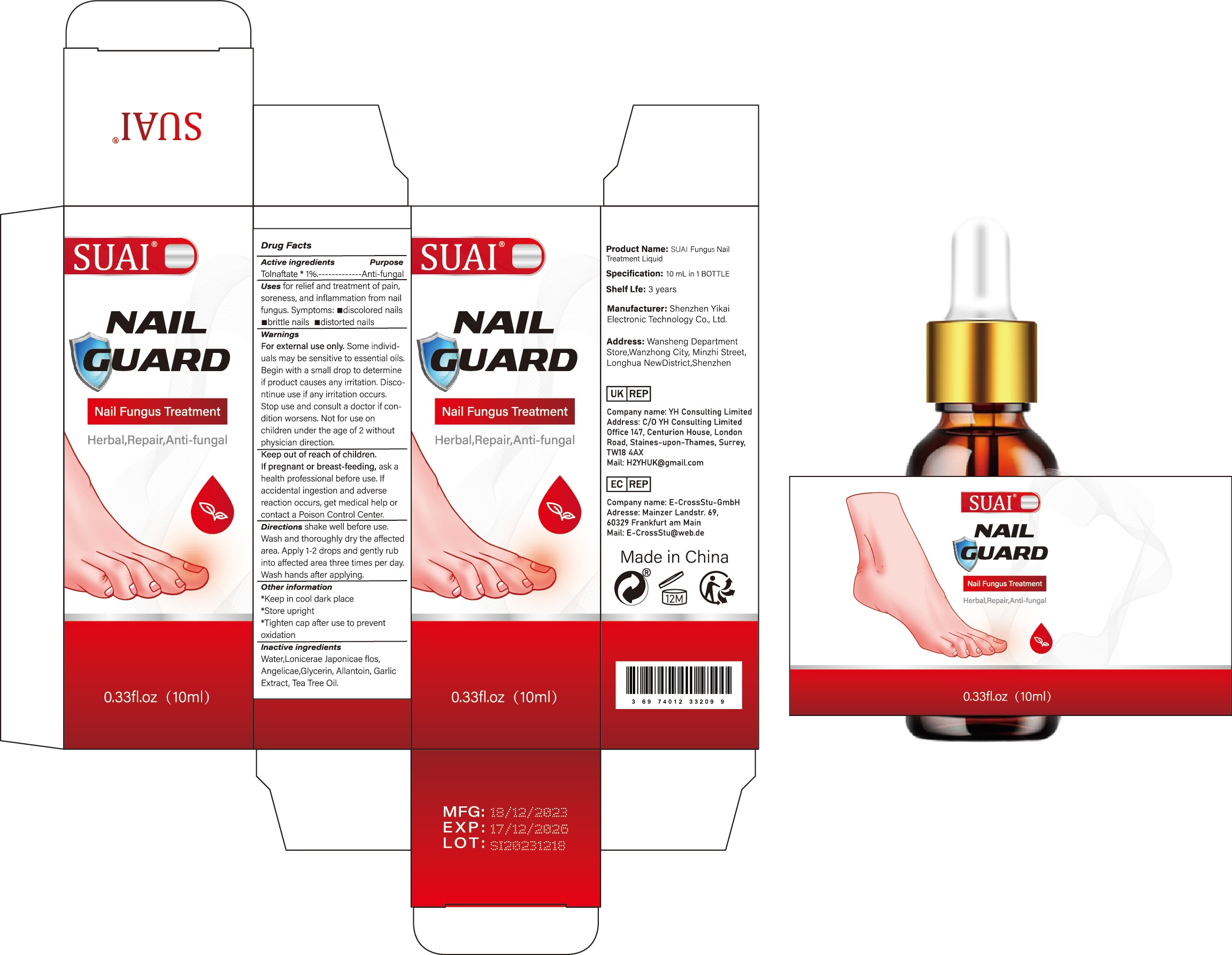
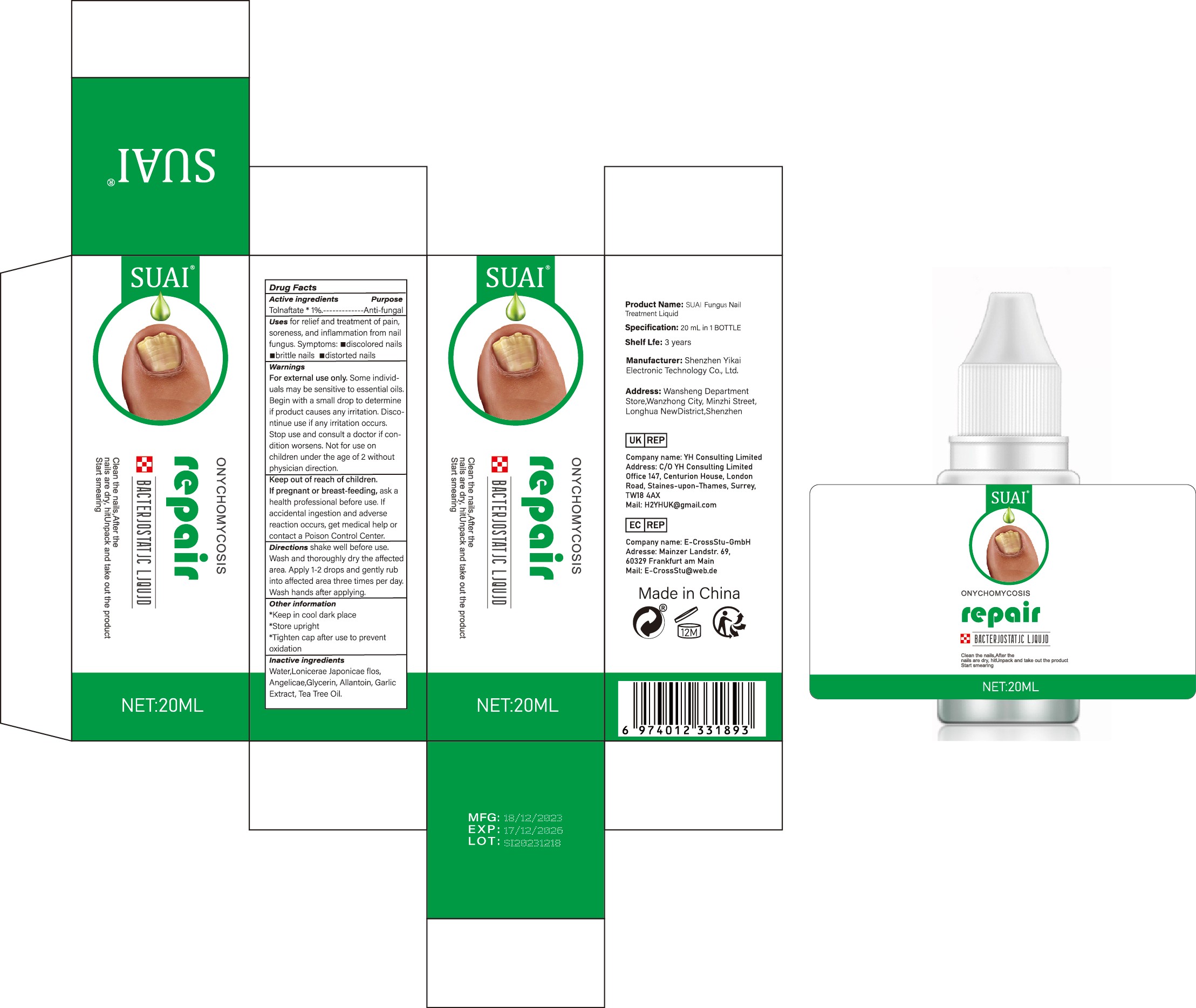
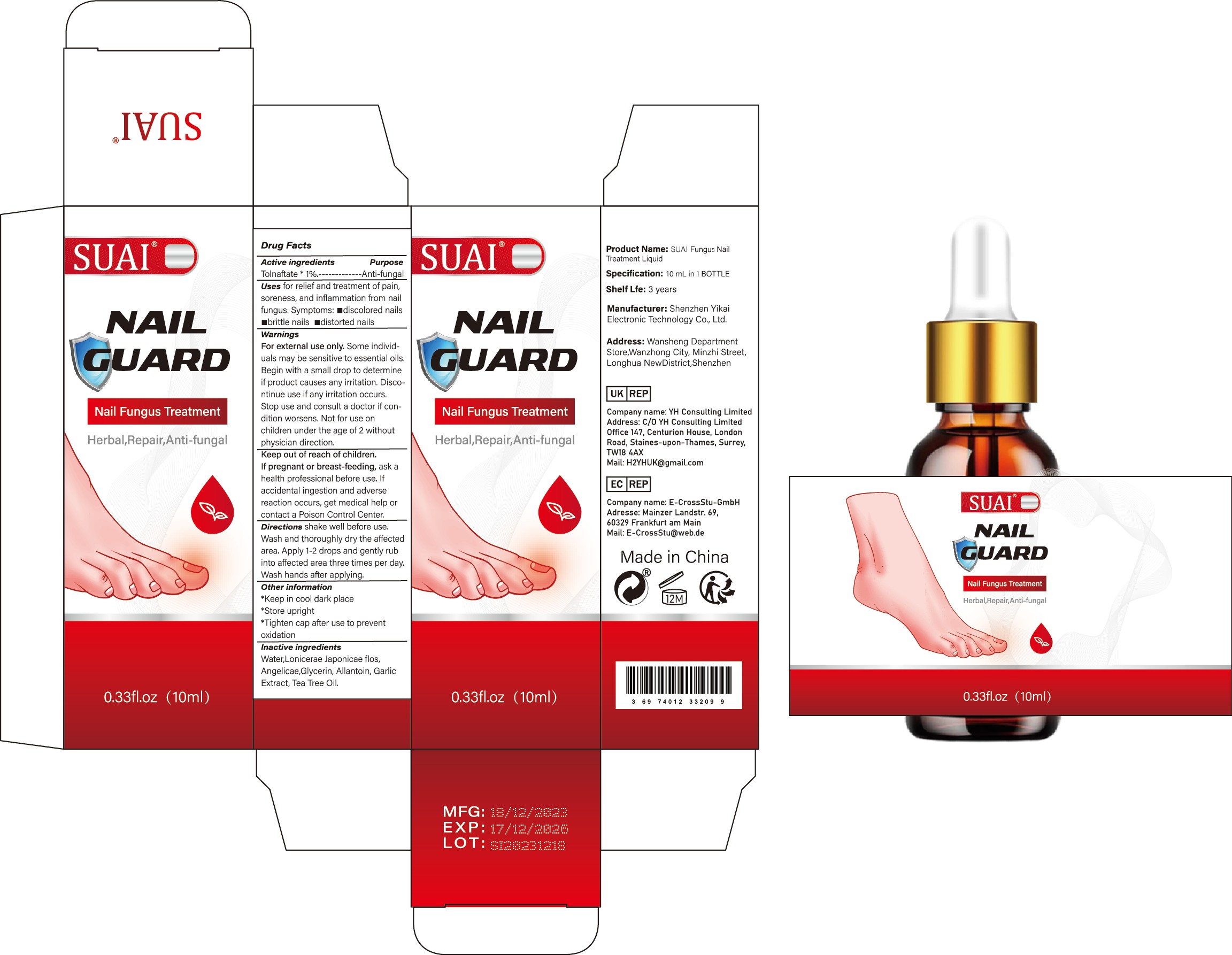
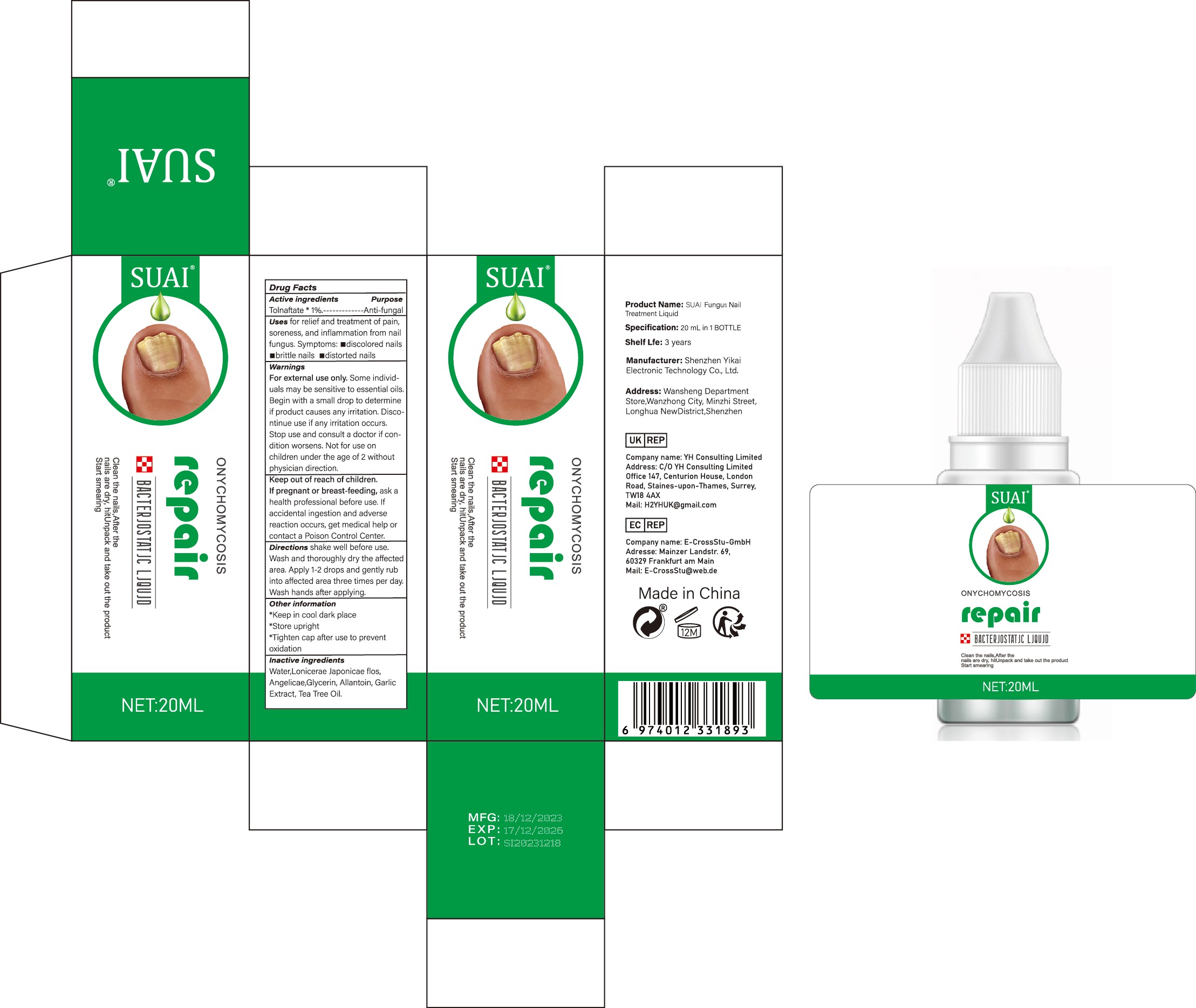
Label: SUAI FUNGUS NAIL TREATMENT LIQUID liquid
- NDC Code(s): 83809-017-01, 83809-017-02
- Packager: Shenzhen Yikai Electronic Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
-
Warnings
For external use only. Some individuals may be sensitive to essential oils. Begin with a small drop to determine if product causes any irritation. Discontinue use if any irritation occurs. Stop use and consult a doctor if condition worsens. Not for use on children under the age of 2 without physician direction.
- Keep Oot Of Reach Of Children
- Directions
- Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SUAI FUNGUS NAIL TREATMENT LIQUID
suai fungus nail treatment liquid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83809-017 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TOLNAFTATE (UNII: 06KB629TKV) (TOLNAFTATE - UNII:06KB629TKV) TOLNAFTATE 1 g in 100 mL Inactive Ingredients Ingredient Name Strength ALLANTOIN (UNII: 344S277G0Z) GARLIC (UNII: V1V998DC17) LONICERA JAPONICA FLOWER (UNII: 4465L2WS4Y) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) TEA TREE OIL (UNII: VIF565UC2G) ANGELICA SINENSIS ROOT (UNII: B66F4574UG) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83809-017-01 10 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/26/2023 2 NDC:83809-017-02 20 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/26/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 12/26/2023 Labeler - Shenzhen Yikai Electronic Technology Co., Ltd. (700426808) Establishment Name Address ID/FEI Business Operations Shenzhen Yikai Electronic Technology Co., Ltd. 700426808 label(83809-017) , manufacture(83809-017)