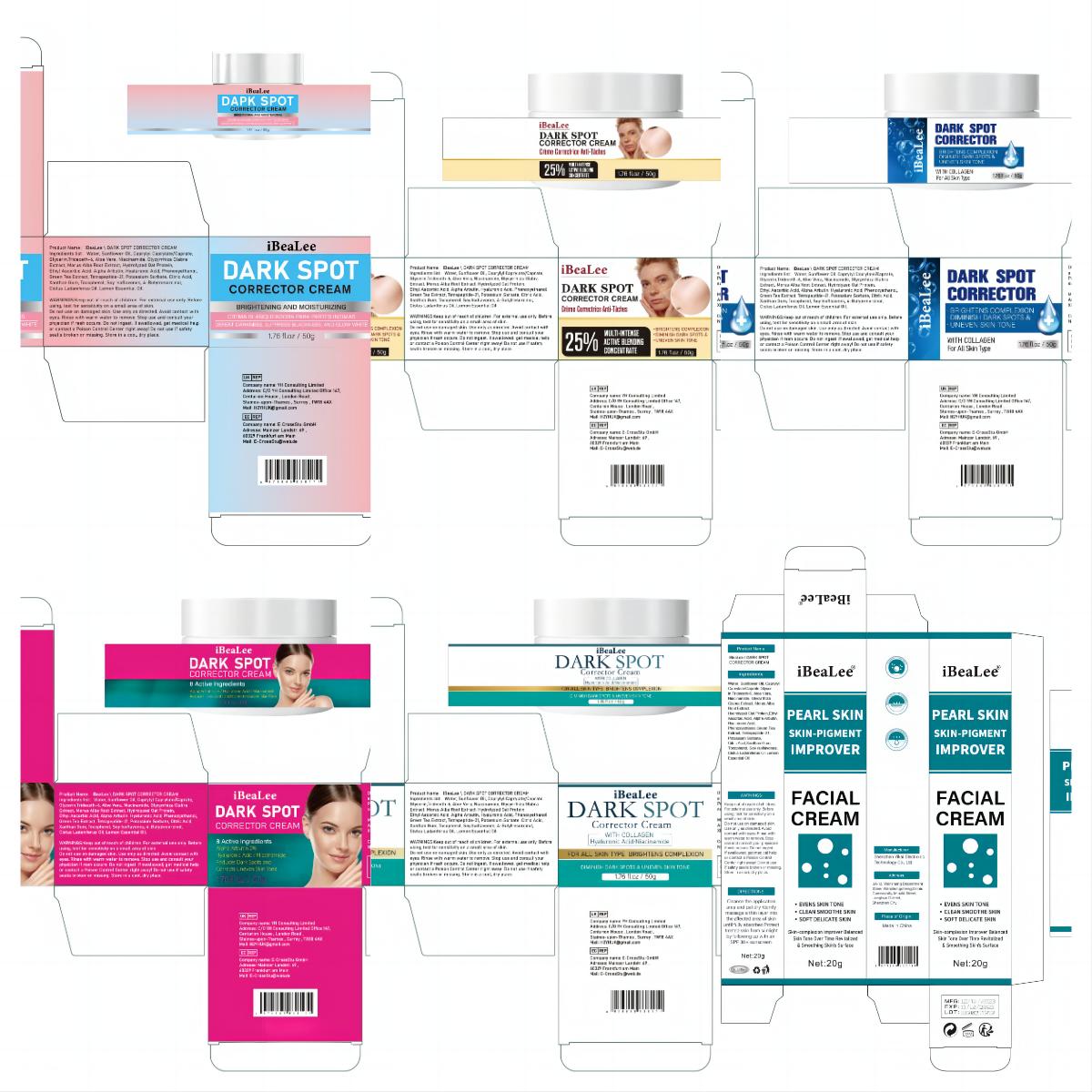
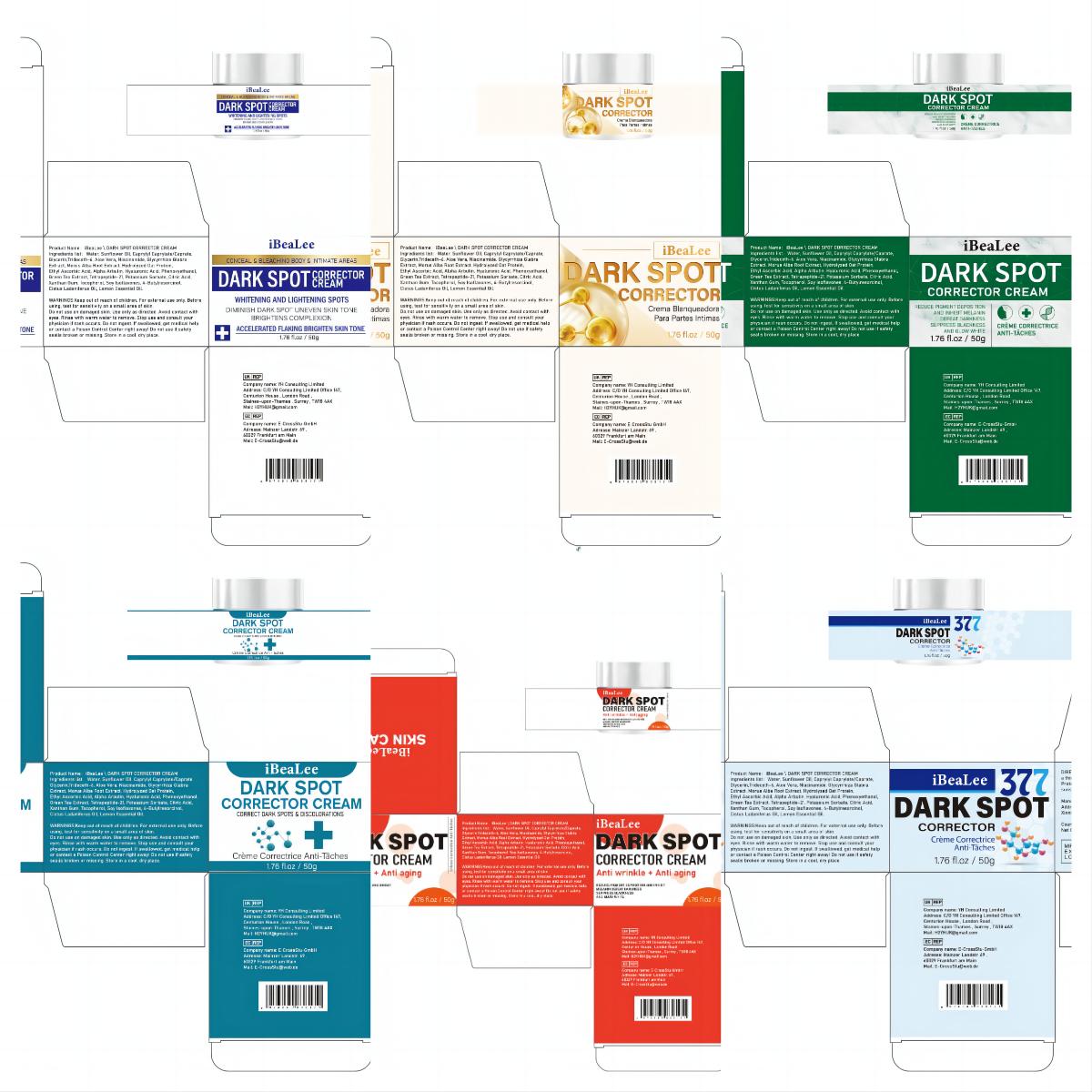
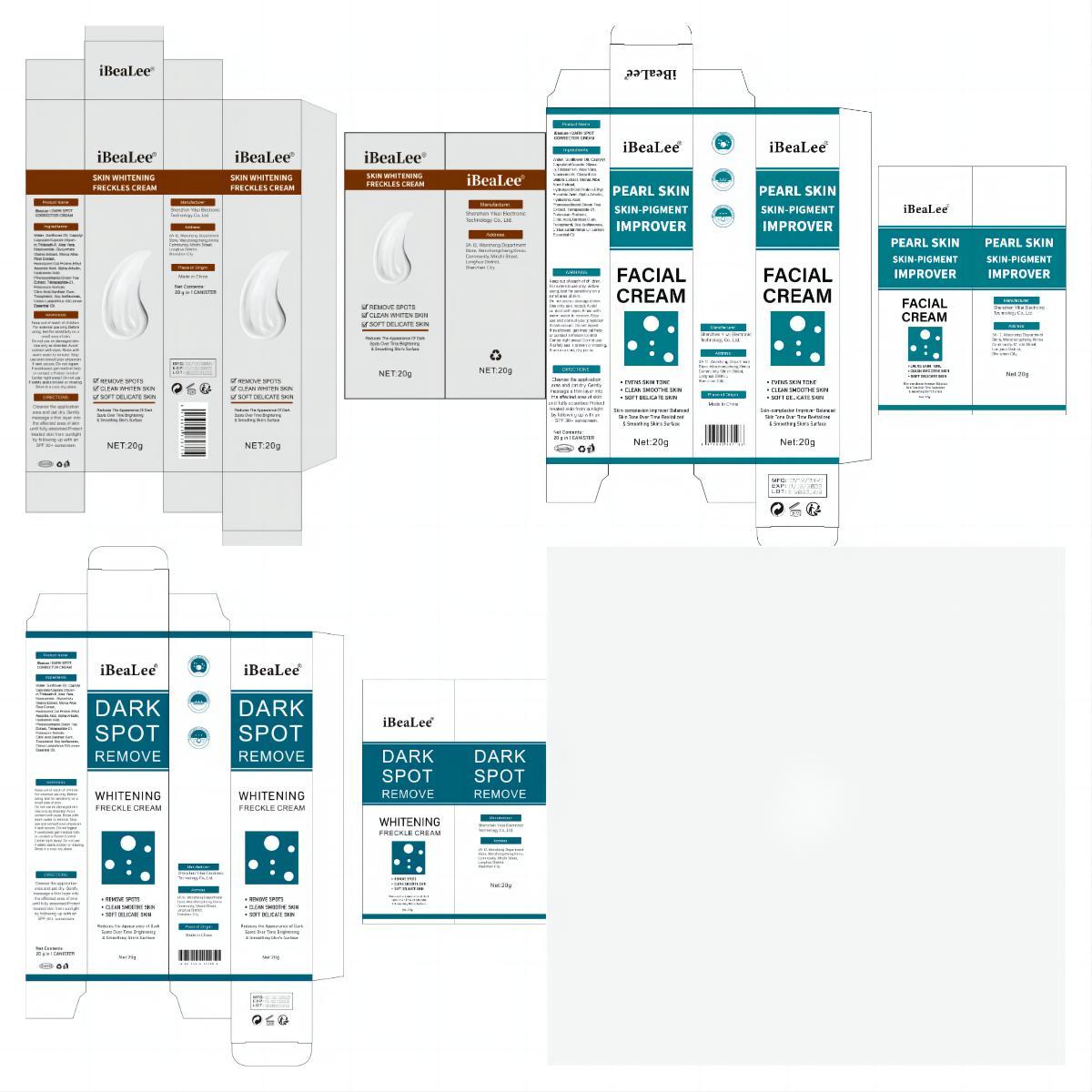
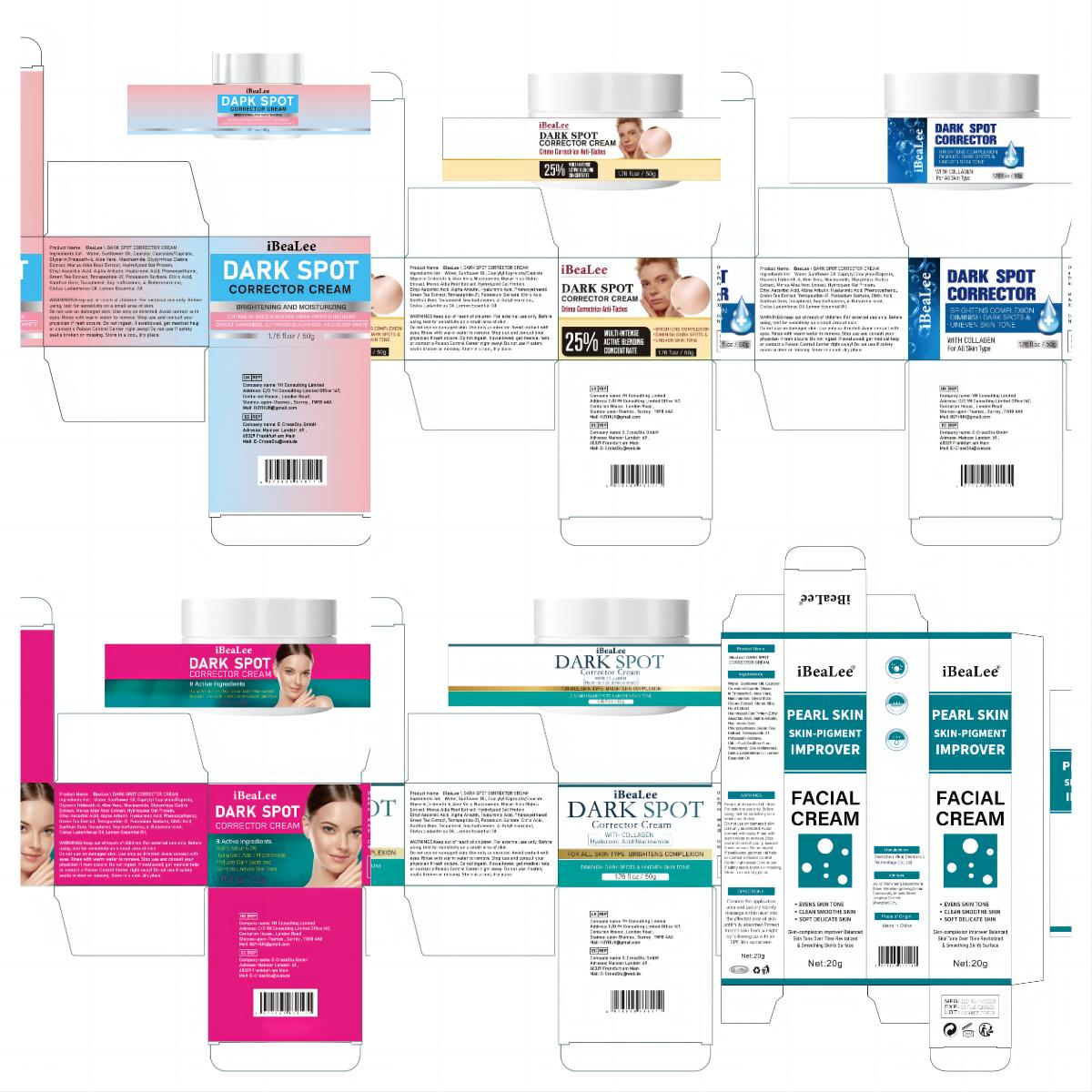
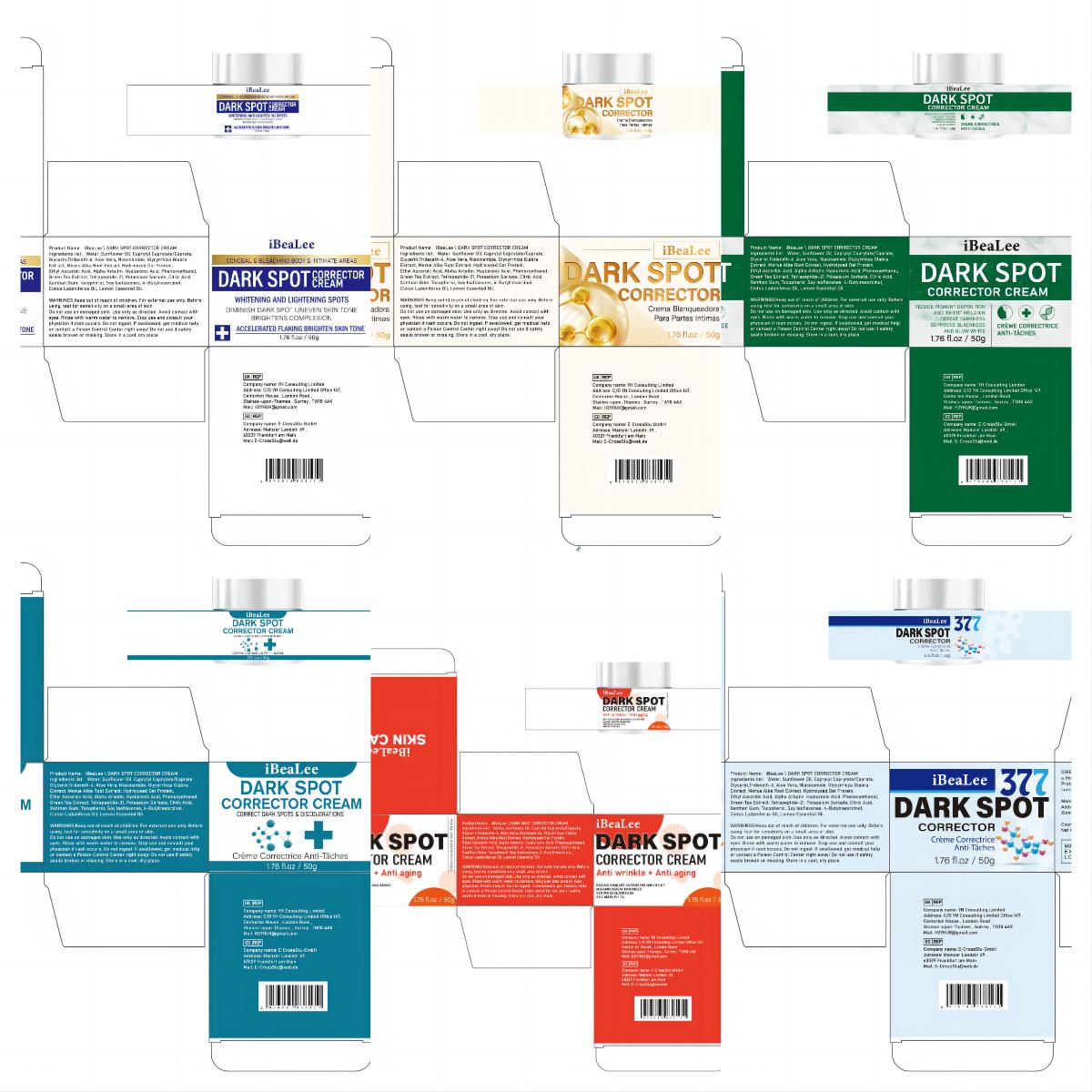
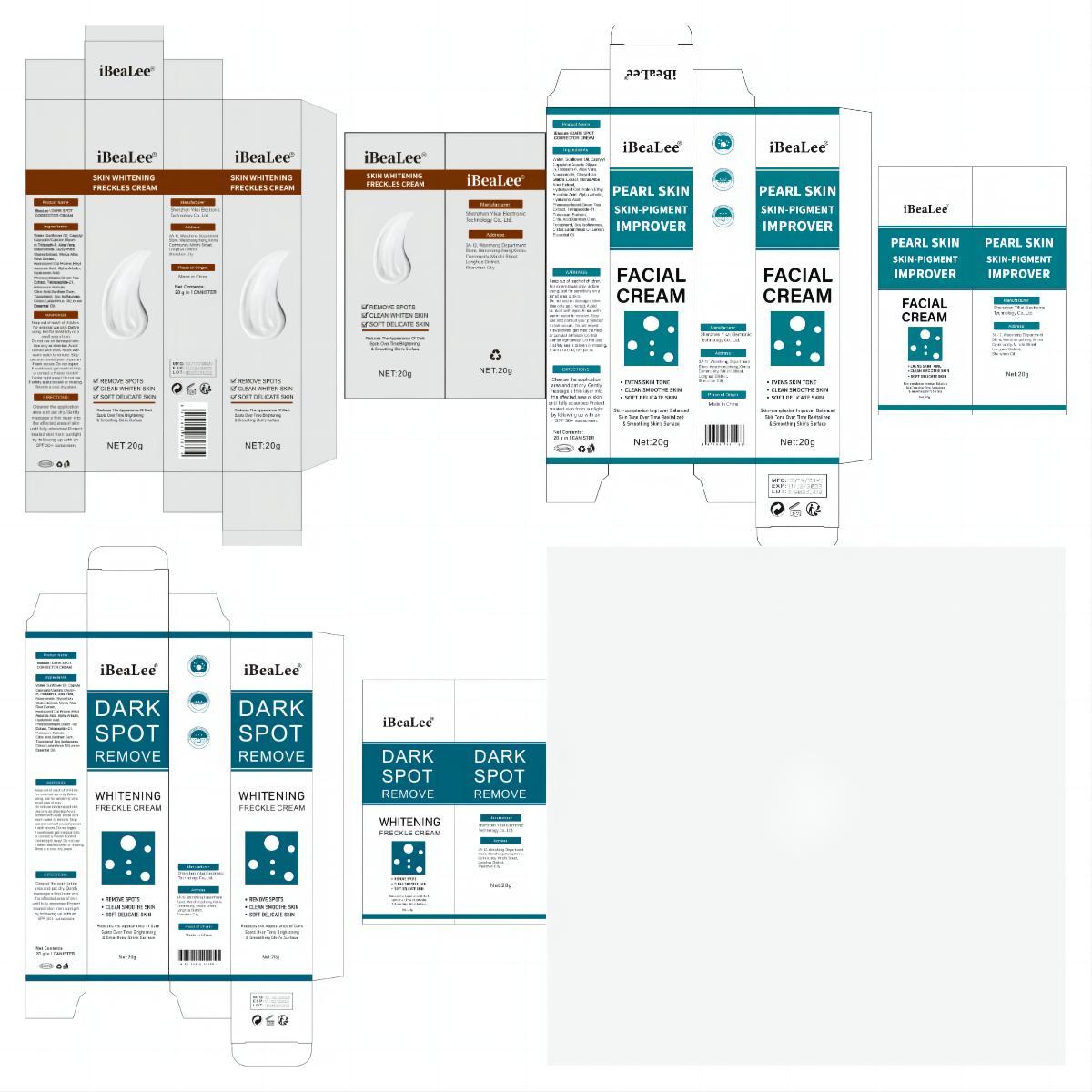
Label: IBEALEE DARK SPOT CORRECTOR CREAM cream
- NDC Code(s): 83809-016-01, 83809-016-02
- Packager: Shenzhen Yikai Electronic Technology Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 27, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Warnings
-
Do not use
Do not use on damaged skin. Use only as directed. Avoid contact with eyes. Rinse with warm water to remove. Stop use and consult your physician if rash occurs. Do not ingest. If swallowed, get medical help or contact a Poison Control Center right away! Do not use if safety sealis broken or missing. Store in a cool, dry place.
- Keep Oot Of Reach Of Children
- Directions
-
Inactive ingredients
Water, Sunflower Oil, Caprylyl Caprylate/Caprate,Glycerin,Trideceth-6, Aloe Vera, Niacinamide, Glycyrrhiza Glabra Extract, Morus Alba Root Extract, Hydrolyzed Oat Protein,Ethyl Ascorbic Acid, Alpha Arbutin, Hyaluronic Acid, Phenoxyethanol,Green Tea Extract, Tetrapeptide-21, Potassium Sorbate, Citric Acid,Xanthan Gum, Tocopherol, Soy Isoflavones, Cistus Ladaniferus Oil,Lemon Essential Oil.
- Use
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
IBEALEE DARK SPOT CORRECTOR CREAM
ibealee dark spot corrector cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:83809-016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 5 g in 100 g Inactive Ingredients Ingredient Name Strength SUNFLOWER OIL (UNII: 3W1JG795YI) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) TRIDECETH-6 (UNII: 3T5PCR2H0C) XANTHAN GUM (UNII: TTV12P4NEE) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 3-O-ETHYL ASCORBIC ACID (UNII: 6MW60CB71P) ALPHA-ARBUTIN (UNII: 72VUP07IT5) PHENOXYETHANOL (UNII: HIE492ZZ3T) GREEN TEA LEAF (UNII: W2ZU1RY8B0) TOCOPHEROL (UNII: R0ZB2556P8) GLYCERIN (UNII: PDC6A3C0OX) GLYCYRRHIZA GLABRA (UNII: 2788Z9758H) MORUS ALBA ROOT (UNII: CST1G9BZGD) CAPRYLYL CAPRYLATE/CAPRATE (UNII: 22MCG4RSMR) GELATIN HYDROLYSATE (PORCINE SKIN, MW 3000) (UNII: 0K9R94573C) HYALURONIC ACID (UNII: S270N0TRQY) TETRAPEPTIDE-21 (UNII: 179JUC43HU) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:83809-016-01 20 g in 1 CANISTER; Type 0: Not a Combination Product 12/26/2023 2 NDC:83809-016-02 50 g in 1 CANISTER; Type 0: Not a Combination Product 12/26/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 12/26/2023 Labeler - Shenzhen Yikai Electronic Technology Co., Ltd. (700426808) Establishment Name Address ID/FEI Business Operations Shenzhen Yikai Electronic Technology Co., Ltd. 700426808 label(83809-016) , manufacture(83809-016)