Label: PROPEL MINI SINUS IMPLANT WITH STRAIGHT DELIVERY SYSTEM- kit
- NHRIC Code(s): 10599-004-01
- Packager: Intersect ENT, INC.
- Category: MEDICAL DEVICE
- DEA Schedule: None
- Marketing Status: Premarket Application
Drug Label Information
Updated December 28, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DEVICE DESCRIPTION
The PROPEL Mini Sinus Implant with Straight Delivery System is a bioabsorbable implant designed to maintain patency of the sinus cavity. The sinus implant is manufactured from a synthetic bioabsorbable copolymer, poly (L-lactide-co-glycolide) (PLG). The implant contains mometasone furoate (active ingredient), a synthetic corticosteroid with anti-inflammatory activity. Mometasone furoate is a white to off-white powder. The chemical name is 9α,21-dichloro-11β,17α-dihydroxy-16α-methylpregna-1,4-diene-3,20-dione 17-(2-furoate), with the empirical formula C27H30Cl2O6, and a molecular weight of 521.43 g/mol. Mometasone furoate is a hydrophobic drug that is practically insoluble in water. Mometasone furoate is stable under aqueous, acidic and oxidative conditions. Mometasone furoate can degrade under extreme basic, thermal and photolytic conditions. The chemical structure is shown. The drug is embedded in a bioabsorbable polymer matrix containing poly-(DL-lactide-co-glycolide) and polyethylene glycol (inactive ingredients) which provides for gradual release of the drug.
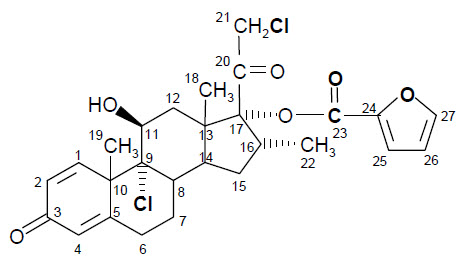
Chemical structure of mometasone furoate
The inactive ingredients on the sinus implant are poly-(DL-lactide-co-glycolide) and polyethylene glycol. Poly-(DL-lactide-co-glycolide) is an amorphous biodegradable polymer. The chemical structure is shown below.
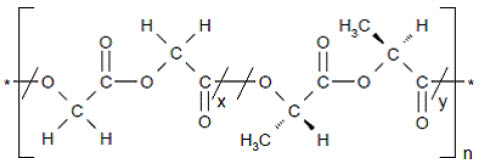
Chemical structure of poly-(DL-lactide-co-glycolide)
Polyethylene glycol is a hydrophilic polyether compound that is highly flexible. It is non-toxic and non-immunogenic. The chemical structure is shown below.
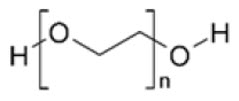
Chemical structure of polyethylene glycol
The PROPEL Mini Sinus Implant with Straight Delivery System is designed to accommodate the size and variability of the post-surgical ethmoid anatomy. The implant is designed to be inserted by a physician under endoscopic visualization and once inserted, the implant is designed to be self-retaining against the mucosa of the surgically enlarged sinus. A delivery system is provided to access the ethmoid sinus and insert the implant.
-
INDICATIONS FOR USE
The PROPEL Mini Sinus Implant with Straight Delivery System is intended for use in patients ≥ 18 years of age following ethmoid sinus surgery to maintain patency of the ethmoid sinus. The PROPEL Mini Sinus Implant separates/dilates surrounding mucosal tissues, provides stabilization of the middle turbinate, prevents obstruction by adhesions, and reduces inflammation. The implant reduces the need for post-operative intervention such as surgical adhesion lysis and/or use of oral steroids.
- CONTRAINDICATIONS
-
WARNINGS AND PRECAUTIONS
WARNINGS
•The PROPEL Mini Sinus Implant with Straight Delivery System is designed for single patient use only. Do not reprocess or reuse.
•Do not use if the package is open or damaged.PRECAUTIONS
- •
- Special care should be taken to avoid bending, twisting or damaging the implant.
- •
- The implant is not designed to be modified by the physician.
- •
- The implant is not intended to be compressed and loaded into the delivery system more than two times.
- •
- The implant must be placed under endoscopic visualization.
- •
- The implant exhibits no antimicrobial properties.
- •
- Foreign body reaction may occur as is possible with most surgical adjuncts.
- •
- In rare instances, the physiochemical condition associated with sinus surgery, both with and without sinus implants or packing, may present a risk of toxic shock syndrome (TSS).
- •
- Pediatric Use: The safety and effectiveness of the implant in pediatric patients have not been established.
- •
- Pregnancy and Nursing Females: The safety and effectiveness of the implant in pregnant or nursing females have not been established.
DRUG INFORMATION
MECHANISM OF ACTION: Corticosteroids have been shown to have a wide range of effects on multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation. The precise mechanism behind the anti-inflammatory properties of the eluted mometasone furoate is not known.
PHARMACOKINETICS: The Propel sinus implant underwent pharmacokinetics testing. Following bilateral drug-eluting Propel implant placement after sinus surgery for chronic sinusitis and subsequent weekly morning blood sampling for 4 weeks in 5 adult patients, plasma mometasone furoate concentrations were not quantifiable at any time point. Mean cortisol concentrations were within normal limits.DRUG INTERACTIONS
No drug-drug interaction studies have been conducted with the PROPEL Mini implant.
CARCINOGENICITY, GENOTOXICITY AND REPRODUCTIVE TOXICITY
No long term studies in animals have been performed to evaluate the carcinogenic potential of the PROPEL Mini implant.
- DOSAGE AND ADMINISTRATION
-
I. POTENTIAL ADVERSE EFFECTS OF THE DEVICE ON HEALTH
Potential Adverse Effects: Potential adverse effects associated with the PROPEL Mini sinus implant are anticipated to be similar to those associated with other sinus stents, gels or packing.
Potential adverse effects associated with the PROPEL Mini Sinus Implant with Straight Delivery System include, but may not be limited to:
•Premature displacement of implant or implant fragments
•Swallowing implant or implant fragments
•Pain/pressure/headache may result from the adherence of crusting to, or presence of the implant
•Aspiration of small implant fragments (not observed in clinical trials)
•Foreign body response, including formation of granulation tissuePotential risks or side effects associated with intranasal mometasone furoate include:
•nasal irritation
•hypersensitivity reaction
•intranasal bleeding
•localized infection (bacterial, fungal or viral) in the nose or pharynx
•nasal burning
•nasal dryness
•susceptibility to secondary infections due to bacteria, fungi or viruses
•glaucoma/elevation of intraocular pressure
•cataracts/change in lens opacities
•headache
•pharyngitisPotential risks or general side effects associated with steroids:
•alteration of the HPA axis including growth suppression
•immunosuppression
•hypersensitivity reactions
•headache
•epistaxis
•coughing
•vomiting
•candidiasis
•glaucoma/elevation in intraocular pressure
•cataracts/changes in lens opacities
•arthralgia
•myalgiaManufactured by:
Intersect ENT, Inc.
1555 Adams Drive
Menlo Park, CA 94025
650-641-2100
www.intersectENT.comCustomer Service 1-866-531-6004
CustomerService@intersectENT.com -
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – PROPEL® Mini with Straight Delivery System

Picture of label is representative of label content
PROPEL® Mini with Straight Delivery System
(mometasone furoate implant, 370 µg)
CONTENTS INCLUDE:
One sinus implant (nominal length: 16 mm)
One straight delivery systemCaution: Federal law (US) restricts this product to sale by or on the order of a physician.
Reference Number
60044Read Instructions Prior to Use
For Single Use Only Lot Number
12345678
Sterilized Using Radiation
Contents are STERILE in unopened and undamaged
package
Use By
2025-05-05
yyyy-mm-ddStore at Room Temperature (15-30° C) intersect® ENT
4282023 10:15:17
01453 Rev. F
1555 Adams Drive, Menlo Park, CA 94025
Customer Service 1-866-531-6004
CustomerService@intersectENT.com -
INGREDIENTS AND APPEARANCE
PROPEL MINI SINUS IMPLANT WITH STRAIGHT DELIVERY SYSTEM
drug-eluting sinus stent kitProduct Information Product Type MEDICAL DEVICE Item Code (Source) NHRIC:10599-004 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:10599-004-01 1 in 1 PACKAGE Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 Part 2 1 Part 1 of 2 DELIVERY SYSTEM
drug-eluting sinus stent implantProduct Information Route of Administration NASAL Inactive Ingredients Ingredient Name Strength POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Premarket Application P100044 01/20/2021 Part 2 of 2 PROPEL MINI SINUS IMPLANT WITH STRAIGHT DELIVERY SYSTEM
drug-eluting sinus stent implantProduct Information Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MOMETASONE FUROATE (UNII: 04201GDN4R) (MOMETASONE - UNII:8HR4QJ6DW8) MOMETASONE FUROATE 370 ug Inactive Ingredients Ingredient Name Strength POLY(DL-LACTIC-CO-GLYCOLIC ACID), (50:50; 12000 MW) (UNII: WE369X5600) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Premarket Application P100044 01/20/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Premarket Application P100044 01/20/2021 Labeler - Intersect ENT, INC. (876715355) Establishment Name Address ID/FEI Business Operations Intersect ENT, INC. 876715355 MANUFACTURE

