Label: NEORA INVISIBLOC SUNSCREEN GEL BROAD SPECTRUM SPF40- avobenzone, homosalate, octisalate lotion
- NDC Code(s): 68577-157-01
- Packager: COSMAX USA, CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 25, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
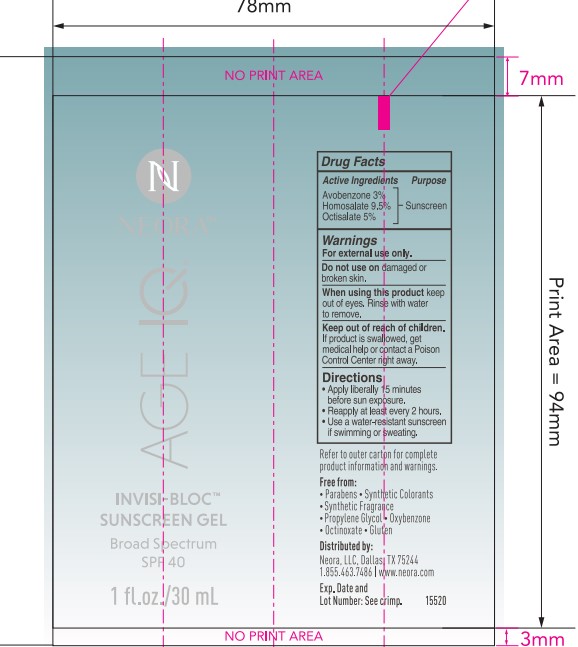
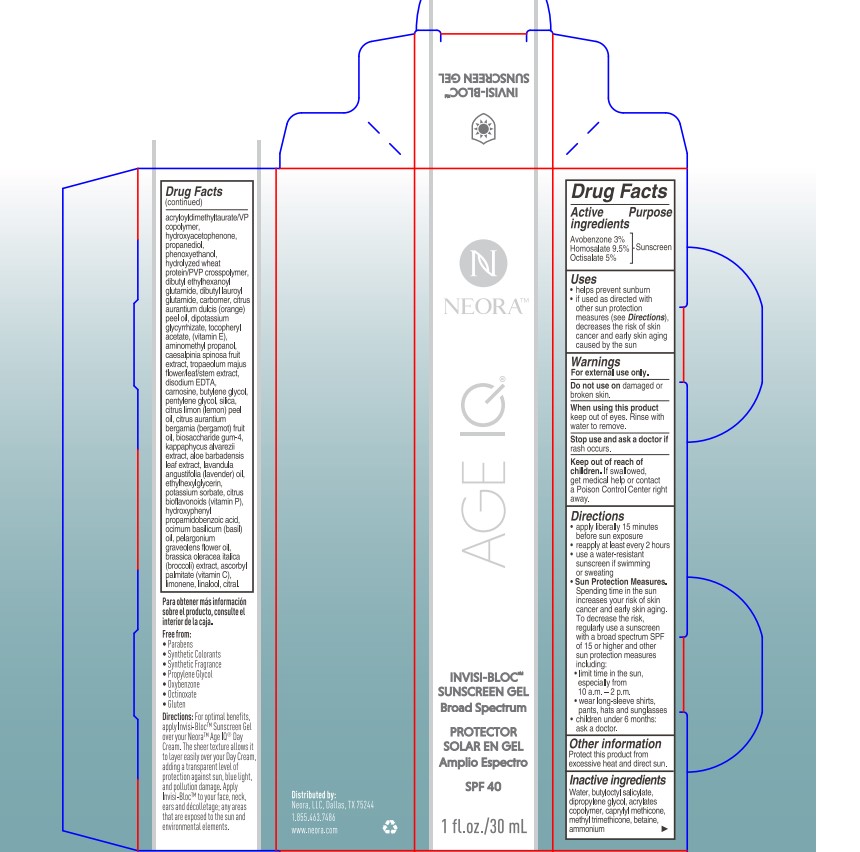
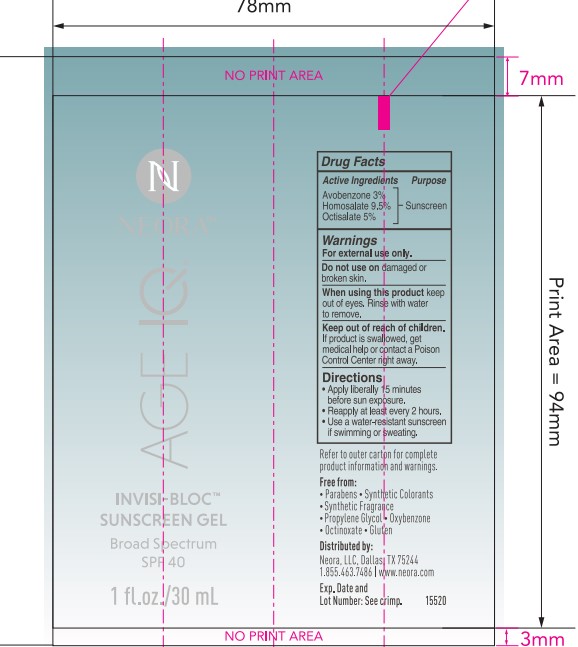
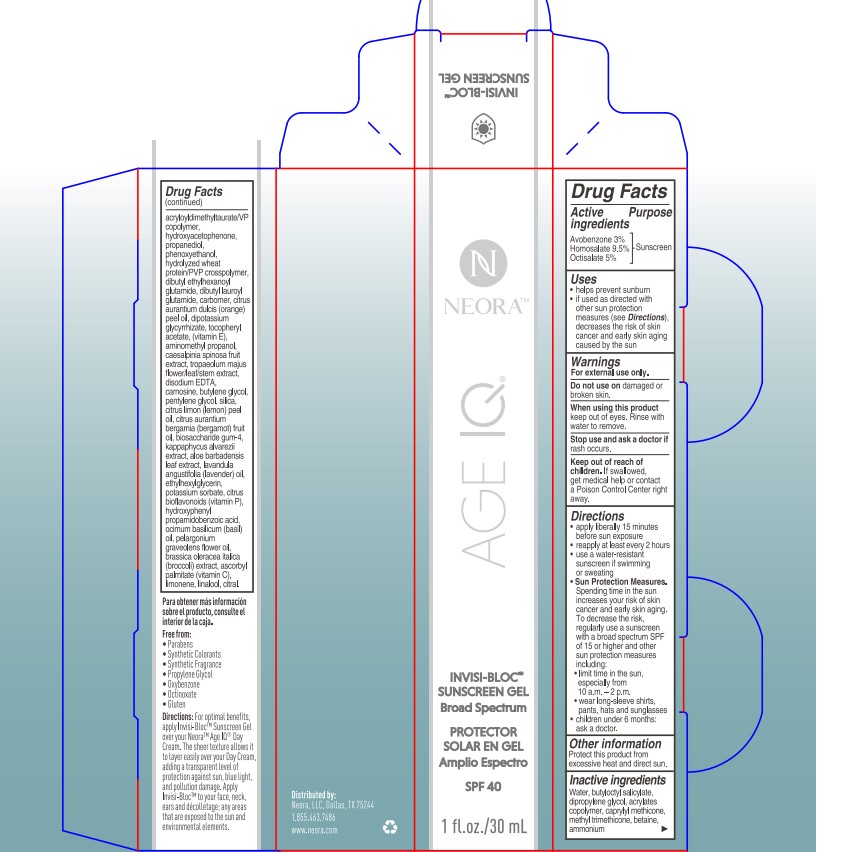
- ACTIVE INGREDIENT
- PURPOSE
- USES
- Warnings
-
DIRECTIONS
Directions
- apply liberrally 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water-resistant sunscreen if swimming or sweating
- Sun Protection Measures.Spending time in the sun
increases your risk of skin cancer and early skin aging. To decrease
this risk, regularly use a sunscreen with a Broad Spectrum SPF
value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age:ask a doctor
- OTHER INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients:
Water, butyloctyl salicylate, dipropylene glycol, acrylates copolymer, caprylyl methicone, methyl trimethicone, betaine, ammonium acryloyldimethyltaurate/VP copolymer, hydroxyacetophenone, propanediol, phenoxyethanol, hydrolyzed wheat protein/PVP crosspolymer, dibutyl ethylhexanoyl glutamide, dibutyl lauroyl glutamide, carbomer, citrus aurantium dulcis (orange) peel oil, dipotassium glycyrrhizate, tocopheryl acetate, (vitamin E), aminomethyl propanol, caesalpinia spinosa fruit extract, tropaeolum majus flower/leaf/stem extract, disodium EDTA, carnosine, butylene glycol, pentylene glycol, silica, citrus limon (lemon) peel oil, citrus aurantium bergamia (bergamot) fruit oil, biosaccharide gum-4, kappaphycus alvarezii extract, aloe barbadensis leaf extract, lavandula angustifolia (lavender) oil, ethylhexylglycerin, potassium sorbate, citrus bioflavonoids (vitamin P), hydroxyphenyl propamidobenzoic acid, ocimum basilicum (basil) oil, pelargonium graveolens flower oil, brassica oleracea italica (broccoli) extract, ascorbyl palmitate (vitamin C), limonene, linalool, citral.
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEORA INVISIBLOC SUNSCREEN GEL BROAD SPECTRUM SPF40
avobenzone, homosalate, octisalate lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68577-157 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 mg in 100 mg HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9.5 mg in 100 mg OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 mg in 100 mg Inactive Ingredients Ingredient Name Strength CITRUS LIMON FLOWERING TOP OIL (UNII: 4C38SS0J60) KAPPAPHYCUS ALVAREZII (UNII: T479H08K2O) CITRAL (UNII: T7EU0O9VPP) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) ASCORBYL PALMITATE (UNII: QN83US2B0N) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) LIMONENE, (+/-)- (UNII: 9MC3I34447) CARNOSINE (UNII: 8HO6PVN24W) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TROPAEOLUM MAJUS FLOWER (UNII: 7FV667B00B) BIOSACCHARIDE GUM-4 (UNII: 9XRL057X90) ALOE VERA LEAF (UNII: ZY81Z83H0X) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PENTYLENE GLYCOL (UNII: 50C1307PZG) HYDROXYPHENYL PROPAMIDOBENZOIC ACID (UNII: 25KRT26H77) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) BROCCOLI SPROUT (UNII: 128UH9LOAE) LINALOOL, (+/-)- (UNII: D81QY6I88E) LAVENDER OIL (UNII: ZBP1YXW0H8) CITRUS BIOFLAVONOIDS (UNII: BD70459I50) WATER (UNII: 059QF0KO0R) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) DIPROPYLENE GLYCOL (UNII: E107L85C40) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) CAPRYLYL TRISILOXANE (UNII: Q95M2P1KJL) METHYL TRIMETHICONE (UNII: S73ZQI0GXM) BASIL OIL (UNII: Z129UMU8LE) BETAINE (UNII: 3SCV180C9W) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) PHENOXYETHANOL (UNII: HIE492ZZ3T) HYDROLYZED WHEAT PROTEIN (ENZYMATIC, 3000 MW) (UNII: J2S07SB0YL) DIBUTYL LAUROYL GLUTAMIDE (UNII: 3V7K3IA58X) CITRUS AURANTIUM FRUIT OIL (UNII: 59JDQ5VT0T) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) PROPANEDIOL (UNII: 5965N8W85T) DIBUTYL ETHYLHEXANOYL GLUTAMIDE (UNII: 0IAF2L30VS) CARBOMER HOMOPOLYMER, UNSPECIFIED TYPE (UNII: 0A5MM307FC) ORANGE OIL (UNII: AKN3KSD11B) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCYRRHIZINATE DIPOTASSIUM (UNII: CA2Y0FE3FX) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) BRASSICA OLERACEA VAR. BOTRYTIS WHOLE (UNII: 87M72T7VW1) AMINOMETHYLPROPANOL (UNII: LU49E6626Q) CAESALPINIA SPINOSA FRUIT POD (UNII: EXY4496LWD) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68577-157-01 1 in 1 CARTON 06/01/2023 1 30 mg in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2023 Labeler - COSMAX USA, CORPORATION (010990210) Registrant - COSMAX USA, CORPORATION (010990210) Establishment Name Address ID/FEI Business Operations COSMAX USA. CORPORATION 010990210 manufacture(68577-157)