Label: EPINASTINE HYDROCHLORIDE solution/ drops
- NDC Code(s): 70069-008-01, 70069-008-10
- Packager: Somerset Therapeutics, LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated November 9, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Epinastine HCl safely and effectively. See full prescribing information for Epinastine HCl.
Epinastine HCl Ophthalmic Solution 0.05%
Initial U.S. Approval: 2003
INDICATIONS AND USAGE
Epinastine HCl Ophthalmic Solution is an H1 histamine receptor antagonist indicated for the prevention of itching associated with allergic conjunctivitis. (1)
DOSAGE AND ADMINISTRATION
The recommended dosage is one drop in each eye twice a day. (2)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution containing 0.5 mg/mL epinastine HCl. (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
The most common ocular adverse reactions (incidence occurring in approximately 1% to 10% of epinastine HCl-treated eyes were burning sensation in the eye, folliculosis, hyperemia, and pruritus. The most common non-ocular adverse reactions, occurring in 10% epinastine HCl-treated eyes, were infection (cold symptoms and upper respiratory infections). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Somerset Therapeutics, LLC at +1 800-417-9175 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Contamination of Tip and Solution
5.2 Use with Contact Lenses
5.3 Topical Ophthalmic Use Only
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy: Teratogenic Effects: Pregnancy Category C
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
17.1 Sterility of Dropper Tip
17.2 Concomitant Use of Contact Lenses
17.3 Topical Ophthalmic Use Only
- *
- Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
- 2 DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Contamination of Tip and Solution
Patients should be instructed to avoid allowing the tip of the dispensing container to contact the eye, surrounding structures, fingers, or any other surface in order to avoid contamination of the solution by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions.
Bottle should be kept tightly closed when not in use.
5.2 Use with Contact Lenses
Patients should be advised not to wear a contact lens if their eye is red. Epinastine HCl ophthalmic solution should not be used to treat contact lens-related irritation.
The preservative in epinastine HCl, benzalkonium chloride, may be absorbed by soft contact lenses. Contact lenses should be removed prior to instillation of epinastine HCl ophthalmic solution and may be reinserted after 10 minutes following its administration.
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
The most frequently reported ocular adverse reactions occurring in approximately 1 to 10% of patients were burning sensation in the eye, folliculosis, hyperemia, and pruritus.
The most frequently reported non-ocular adverse reactions were infection (cold symptoms and upper respiratory infections), seen in approximately 10% of patients, and headache, rhinitis, sinusitis, increased cough, and pharyngitis, seen in approximately 1 to 3% of patients.
Some of these reactions were similar to the underlying disease being studied.
6.2 Postmarketing Experience
The following reactions have been identified during postmarketing use of epinastine HCl in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to epinastine HCl, or a combination of these factors, include: lacrimation increased.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy: Teratogenic Effects: Pregnancy Category C
In an embryofetal developmental study in pregnant rats, maternal toxicity with no embryofetal effects was observed at an oral dose that was approximately 150,000 times the maximum recommended ocular human dose (MROHD) of 0.0014 mg/kg/day on a mg/kg basis. Total resorptions and abortion were observed in an embryofetal study in pregnant rabbits at an oral dose that was approximately 55,000 times the MROHD. In both studies, no drug-induced teratogenic effects were noted.
Epinastine reduced pup body weight gain following an oral dose to pregnant rats that was approximately 90,000 times the MROHD.
There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, epinastine HCl ophthalmic solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
8.3 Nursing Mothers
A study in lactating rats revealed excretion of epinastine in the breast milk. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when epinastine HCl ophthalmic solution is administered to a nursing woman.
-
11 DESCRIPTION
Epinastine HCl Ophthalmic Solution 0.05% is a clear, colorless, sterile isotonic solution containing epinastine HCl, an antihistamine and an inhibitor of histamine release from the mast cell for topical administration to the eyes.
Epinastine HCl is represented by the following structural formula:
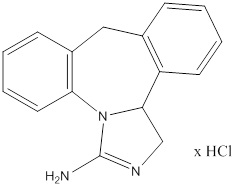
Chemical Name: 3-Amino-9,13b-dihydro-1H-dibenz[c,f]imidazo [1,5-a]azepine hydrochloride
Each mL contains: Active: epinastine HCl 0.05% (0.5 mg/mL) equivalent to epinastine 0.044% (0.44 mg/mL); Preservative: benzalkonium chloride 0.01%; Inactives: edetate disodium; water for injection; sodium chloride; sodium phosphate, monobasic; and sodium hydroxide and/or hydrochloric acid (to adjust pH). Epinastine HCl has a pH of approximately 7 and an osmolality range of 250 to 310 mOsm/kg.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Epinastine is a topically active, direct H1-receptor antagonist and an inhibitor of the release of histamine from the mast cell. Epinastine is selective for the histamine H1-receptor and has affinity for the histamine H2-receptor. Epinastine also possesses affinity for the ɑ1, ɑ2-, and 5-HT2-receptors.
12.3 Pharmacokinetics
Fourteen subjects, with allergic conjunctivitis, received one drop of epinastine HCl ophthalmic solution in each eye twice daily for seven days. On day seven, average maximum epinastine plasma concentrations of 0.04±0.014 ng/ml were reached after about two hours indicating low systemic exposure. While these concentrations represented an increase over those seen following a single dose, the day 1 and day 7 Area Under the Curve (AUC) values were unchanged indicating that there is no increase in systemic absorption with multiple dosing. Epinastine is 64% bound to plasma proteins. The total systemic clearance is approximately 56 L/hr and the terminal plasma elimination half-life is about 12 hours. Epinastine is mainly excreted unchanged. About 55% of an intravenous dose is recovered unchanged in the urine with about 30% in feces. Less than 10% is metabolized. The renal elimination is mainly via active tubular secretion.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In 18-month or 2-year dietary carcinogenicity studies in mice or rats, respectively, epinastine was not carcinogenic at doses up to 40 mg/kg [approximately 30,000 times higher than the MROHD, assuming 100% absorption in humans and animals].
Epinastine in newly synthesized batches was negative for mutagenicity in the Ames / Salmonella assay and in vitro chromosome aberration assay using human lymphocytes. Positive results were seen with early batches of epinastine in two in vitro chromosomal aberration studies conducted in the 1980s with human peripheral lymphocytes and with V79 cells, respectively. Epinastine was negative in the in vivo clastogenicity studies, including the mouse micronucleus assay and chromosome aberration assay in Chinese hamsters. Epinastine was also negative in the cell transformation assay using Syrian hamster embryo cells, V79/HGPRT mammalian cell point mutation assay, and in vivo/in vitro unscheduled DNA synthesis assay using rat primary hepatocytes.
Epinastine had no effect on fertility of male rats. Decreased fertility in female rats was observed at an oral dose up to approximately 90,000 times the MROHD.
-
14 CLINICAL STUDIES
Epinastine HCl 0.05% has been shown to be significantly superior to vehicle for improving ocular itching in patients with allergic conjunctivitis in clinical studies using two different models: (1) conjunctival antigen challenge (CAC) where patients were dosed and then received antigen instilled into the inferior conjunctival fornix; and (2) environmental field studies where patients were dosed and evaluated during allergy season in their natural habitat. Results demonstrated a rapid onset of action for epinastine HCl 0.05% within 3 to 5 minutes after conjunctival antigen challenge. Duration of effect was shown to be 8 hours, making a twice daily regimen suitable. This dosing regimen was shown to be safe and effective for up to 8 weeks, without evidence of tachyphylaxis.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Epinastine HCl ophthalmic solution 0.05% is supplied sterile in opaque white LDPE plastic bottles with dropper tips and white polypropylene caps as follows:
5 mL in 10 mL bottle; individually packaged NDC 70069-008-01
Storage: Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) (See USP Controlled Room Temperature). Keep bottle tightly closed and out of the reach of children.
-
17 PATIENT COUNSELING INFORMATION
17.1 Sterility of Dropper Tip
Patients should be advised not to touch dropper tip to any surface, as this may contaminate the contents (see WARNINGS AND PRECAUTIONS, 5.1)
17.2 Concomitant Use of Contact Lenses
Patients should be advised not to wear a contact lens if their eye is red. Patients should be advised that epinastine HCl should not be used to treat contact lens-related irritation. Patients should also be advised to remove contact lenses prior to instillation of epinastine HCl. The preservative in epinastine HCl ophthalmic solution, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of epinastine HCl.
- SPL UNCLASSIFIED SECTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EPINASTINE HYDROCHLORIDE
epinastine hydrochloride solution/ dropsProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:70069-008 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength EPINASTINE HYDROCHLORIDE (UNII: GFM415S5XL) (EPINASTINE - UNII:Q13WX941EF) EPINASTINE HYDROCHLORIDE 0.5 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) 0.1 mg in 1 mL EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) SODIUM HYDROXIDE (UNII: 55X04QC32I) HYDROCHLORIC ACID (UNII: QTT17582CB) WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (Clear Solution) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70069-008-10 10 in 1 CARTON 09/14/2016 1 NDC:70069-008-01 1 in 1 CARTON 1 5 mL in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA090951 09/14/2016 Labeler - Somerset Therapeutics, LLC (079947873) Registrant - Somerset Therapeutics, LLC (079947873) Establishment Name Address ID/FEI Business Operations Somerset Therapeutics Limited 677236695 ANALYSIS(70069-008) , LABEL(70069-008) , MANUFACTURE(70069-008) , PACK(70069-008)




