Label: BLOWFISH- aspirin, caffeine tablet, effervescent
-
NDC Code(s):
75920-0464-1,
75920-0464-2,
75920-0464-4,
75920-0464-5, view more75920-0464-6, 75920-0464-7, 75920-0464-8
- Packager: Rally Labs LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 29, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
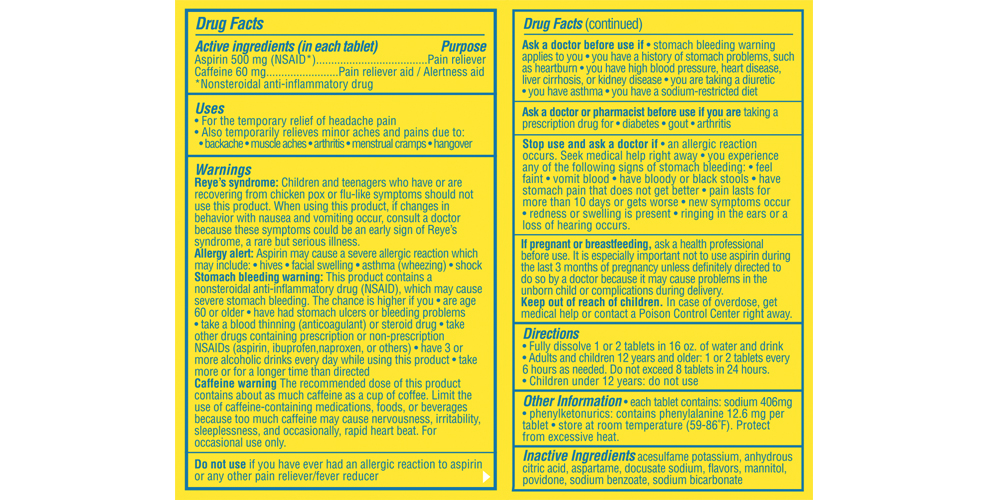
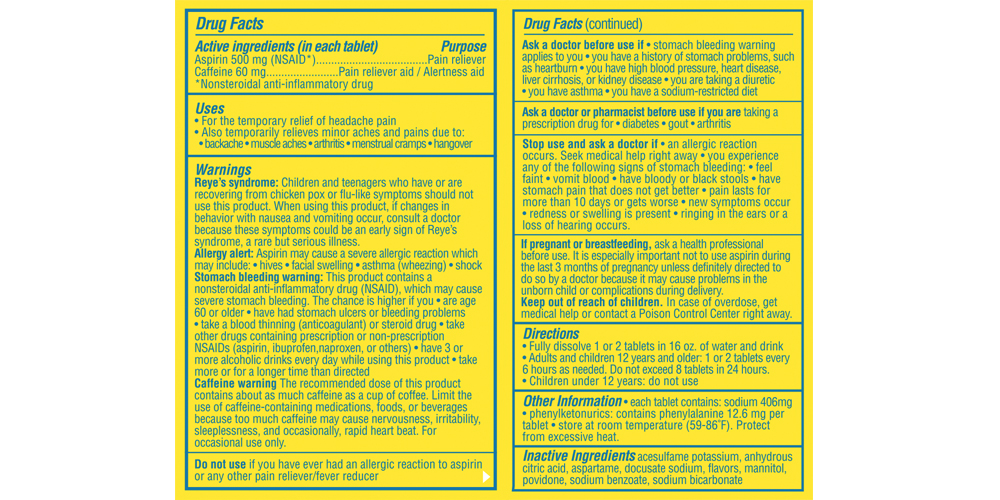
- Active ingredients
- Purpose
-
Uses
- for the temporary relief of minor aches and pains associated with a hangover
- helps restore mental alertness or wakefulness when experiencing fatigue or drowsiness associated with a hangover
- also for the temporary relief of headaches or body aches and pains alone
- for the temporary relief of headache pain
- also temporarily relieves minor aches and pains due to: backache muscle aches arthritis menstrual cramps hangover
- for the temporary relief of muscle aches and pain
- helps restore mental alertness when experiencing fatigue
-
Warnings
Reye’s syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product. If changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reyes syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning:
This product contains a nonsteroidal anti-inflammatory drug (NSAID) which may cause severe stomach bleeding. The chance is higher if you- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or non-prescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
- Do not use
- Ask a doctor before use if
- Ask a doctor or phamacist before use if you are
-
Caffeine warning
-
the recommended dose of this product contains about as much caffeine as a cup of coffee. Limit the use of caffeine-containing medications, foods, or beverages because too much caffeine may cause nervousness, irritability, irritability, sleeplessness, and occasionally rapid heart beat.
- for occasional use only. Do not use for more than 2 days for a hangover unless directed by a doctor. Not intended for use as a substitute for sleep. If fatigue or drowsiness persists or continues to occur, consult a doctor.
-
-
Stop use and ask a doctor if
- an allergic reaction occurs. Seek medical help right away.
-
you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
-
pain lasts for more than 10 days or gets worse
-
new symptoms occur
-
redness or swelling is present
-
ringing in the ears or a loss of hearing occurs
- If pregnant or breastfeeding
- Keep out of reach of children
-
Directions
- upon waking, fully dissolve 2 tablets in 16 oz. of water and drink
- do not exceed recommended dosage
Adults and children 12 years and over
(up to 60 years of age)2 tablets every 6 hours, as needed, or as directed by a doctor. Do not exceed 8 tablets in 24 hours Adults 60 years and over 2 tablets every 6 hours, as needed, or as directed by a doctor. Do not exceed 4 tablets in 24 hours Children under 12 years Do not use - Other information
- Inactive ingredients
- Questions or Comments?
-
Blowfish for Hangovers
Blowfish for Hangovers
Alertness Aid - Caffeine
Pain Reliever - Aspirin (NSAID)
12 effervescent tabletsLemon Flavor
12_Count_Box_Outside_Revised_12_27.jpg
IMAGE NAME IMAGE DELETE IMAGE REFERENCED
12_Count_Box_Outside_Revised_12_27.jpg [Delete Image] Yes
product--12Tablet.jpg

-
INGREDIENTS AND APPEARANCE
BLOWFISH
aspirin, caffeine tablet, effervescentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75920-0464 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASPIRIN (UNII: R16CO5Y76E) (ASPIRIN - UNII:R16CO5Y76E) ASPIRIN 500 mg CAFFEINE (UNII: 3G6A5W338E) (CAFFEINE - UNII:3G6A5W338E) CAFFEINE 60 mg Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ASPARTAME (UNII: Z0H242BBR1) DOCUSATE SODIUM (UNII: F05Q2T2JA0) MANNITOL (UNII: 3OWL53L36A) POVIDONE (UNII: FZ989GH94E) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM BICARBONATE (UNII: 8MDF5V39QO) Product Characteristics Color white Score no score Shape ROUND Size 25mm Flavor LEMON Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75920-0464-1 2 in 1 BOX; Type 0: Not a Combination Product 07/23/2011 2 NDC:75920-0464-8 12 in 1 BOX; Type 0: Not a Combination Product 07/23/2011 3 NDC:75920-0464-2 20 in 1 BAG; Type 0: Not a Combination Product 11/30/2017 4 NDC:75920-0464-4 40 in 1 BAG; Type 0: Not a Combination Product 11/30/2017 5 NDC:75920-0464-6 4 in 1 BOX; Type 0: Not a Combination Product 02/01/2020 6 NDC:75920-0464-5 20 in 1 BOX; Type 0: Not a Combination Product 12/28/2020 7 NDC:75920-0464-7 20 in 1 BOX; Type 0: Not a Combination Product 12/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 07/23/2011 Labeler - Rally Labs LLC (965453108) Establishment Name Address ID/FEI Business Operations Tower Laboratories Ltd 001587203 manufacture(75920-0464)