Label: BUBBLE SOLAR MATE DAILY MINERAL SUNSCREEN SPF 40- zinc oxide cream
- NDC Code(s): 68577-156-01
- Packager: COSMAX USA, CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USES
- Warnings
-
DIRECTIONS
Directions
- Apply liberally 15 minutes before sun exposure
- Reapply at least every 2 hours
- Use a water resistant sunscreen if swimming or sweating
- Children under 6 months of age: Ask a doctor
Sun Protection Measures
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear long-sleeved shirts, pants, hats, and sunglasses.
- OTHER INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredientsWater (Aqua), Isoamyl Laurate, Glycerin, Caprylic/Capric Triglyceride, Butyloctyl Salicylate, Polyglyceryl-10 Stearate, Poly C10-30 Alkyl Acrylate, Polyhydroxystearic Acid, Cetearyl Olivate, Sorbitan Olivate, Rubus Idaeus (Raspberry) Seed Oil, Panax Ginseng Root Extract, Theobroma Cacao (Cocoa) Seed Extract, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Hydroxyacetophenone, Polyglyceryl-10 Laurate, Microcrystalline Cellulose, Butylene Glycol, Xanthan Gum, Cellulose Gum, Phenoxyethanol.
- QUESTIONS or COMMENTS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BUBBLE SOLAR MATE DAILY MINERAL SUNSCREEN SPF 40
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68577-156 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 12 mg in 100 mg Inactive Ingredients Ingredient Name Strength ISOAMYL LAURATE (UNII: M1SLX00M3M) GLYCERIN (UNII: PDC6A3C0OX) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POLYGLYCERYL-10 STEARATE (UNII: 90TF85HH91) BEHENYL ACRYLATE POLYMER (UNII: D64PM5UT4U) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) CETEARYL OLIVATE (UNII: 58B69Q84JO) SORBITAN OLIVATE (UNII: MDL271E3GR) RASPBERRY SEED OIL (UNII: 9S8867952A) ASIAN GINSENG (UNII: CUQ3A77YXI) COCOA (UNII: D9108TZ9KG) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (45000 MPA.S AT 1%) (UNII: 86FQE96TZ4) WATER (UNII: 059QF0KO0R) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) POLYGLYCERYL-10 LAURATE (UNII: MPJ2Q8WI8G) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) XANTHAN GUM (UNII: TTV12P4NEE) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68577-156-01 1 in 1 CARTON 06/01/2023 1 50 mg in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2023 Labeler - COSMAX USA, CORPORATION (010990210) Registrant - COSMAX USA, CORPORATION (010990210) Establishment Name Address ID/FEI Business Operations COSMAX USA. CORPORATION 010990210 manufacture(68577-156)


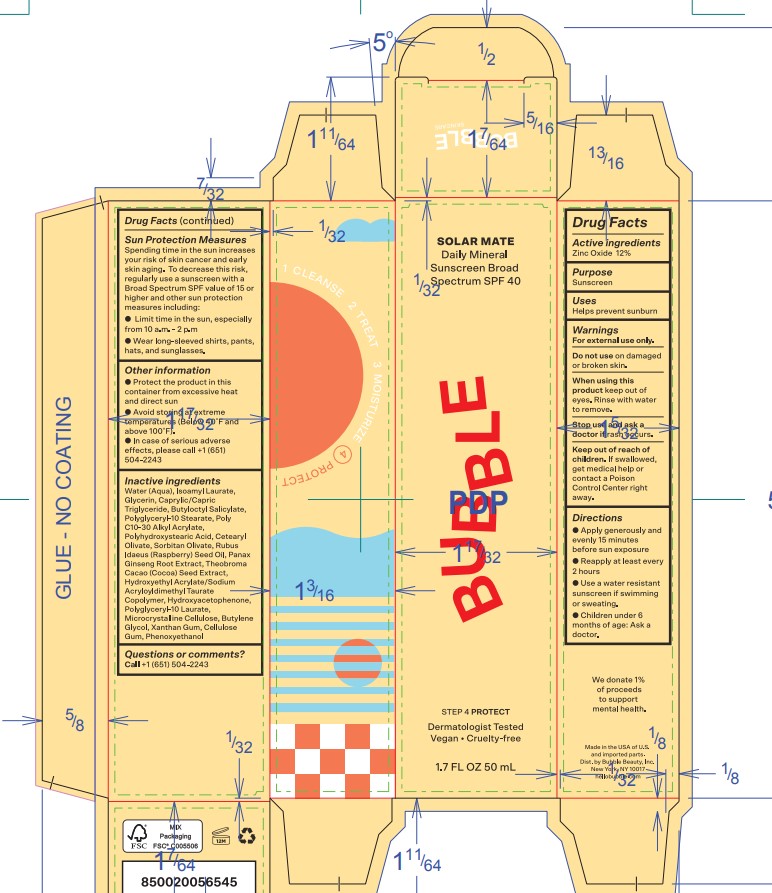
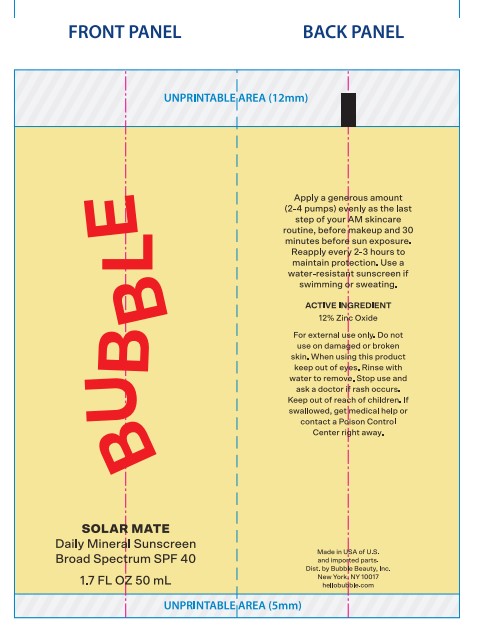 Primary Package(Inner)
Primary Package(Inner)
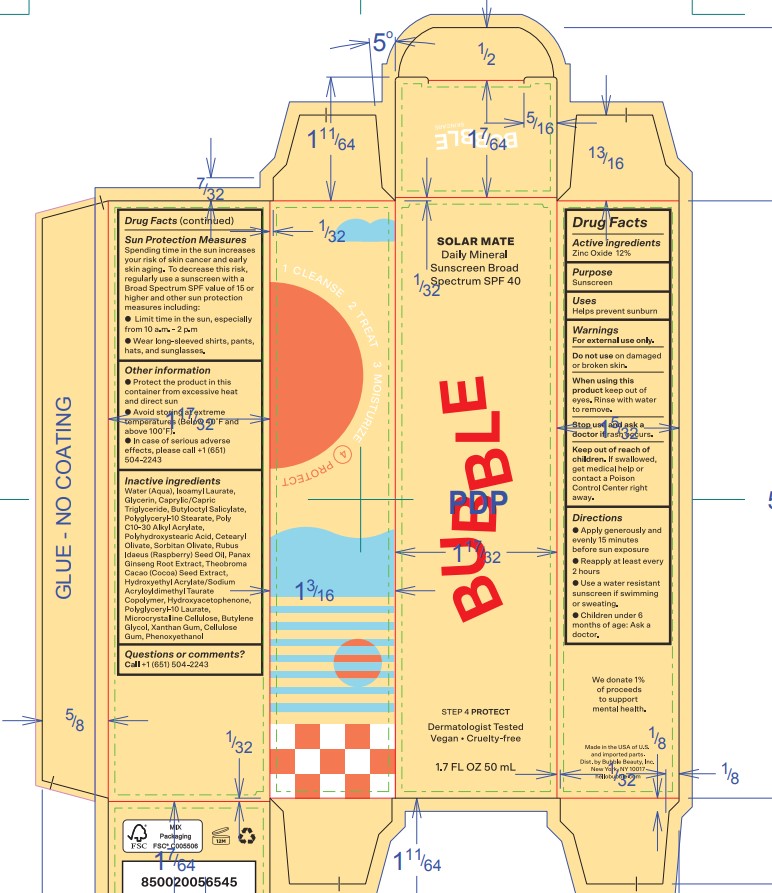 Outer Package (Secondary)
Outer Package (Secondary)