Label: MORPHE SPF 30 BROAD SPECTRUM SUNSETTER SETTING- octisalate, octocrylene, avobenzone, homosalate spray
- NDC Code(s): 68577-151-01
- Packager: COSMAX USA, CORPORATION
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 23, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- PURPOSE
- USES
- Warnings
-
DIRECTIONS
Directions
- Apply evenly 15 minutes before sun exposure
- Reapply
- Reapply at least every 2 hours, or as needed
- Use a water resistant sunscreen if swimming or sweating
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2p.m.
-wear long-sleeved shirts, pants, hats and sunglasses
Children under 6 months of age: Ask a doctor
- Apply evenly 15 minutes before sun exposure
- OTHER INFORMATION
-
INACTIVE INGREDIENT
Inactive ingredients: Alcohol Denat., Isododecane, Butyloctyl Salicylate, PVP, Fragrance, Benzyl Benzoate, Ethylcellulose, Sodium Acetate, Glycine Soja (Soybean) Oil, Polyglyceryl-3 Diisostearate, Oryza Sativa (Rice) Extract, Oryza Sativa (Rice) Germ Extract, Water, Glycerin, Cereus Grandiflorus (Cactus) Flower Extract, Potassium Sorbate, Phenoxyethanol, Citric Acid, Sodium Benzoate, Tropaeolum Majus Flower/Leaf/Steam Extract, Ethylhexylglycerin.
- QUESTIONS or COMMENTS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MORPHE SPF 30 BROAD SPECTRUM SUNSETTER SETTING
octisalate, octocrylene, avobenzone, homosalate sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68577-151 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2 mg in 100 mg HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9 mg in 100 mg OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2 mg in 100 mg OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 mg in 100 mg Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) ISODODECANE (UNII: A8289P68Y2) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POVIDONE (UNII: FZ989GH94E) FRAGRANCE 13576 (UNII: 5EM498GW35) BENZYL BENZOATE (UNII: N863NB338G) ETHYLCELLULOSES (UNII: 7Z8S9VYZ4B) SODIUM ACETATE (UNII: 4550K0SC9B) SOYBEAN OIL (UNII: 241ATL177A) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) RICE GERM (UNII: 7N2B70SFEZ) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) SELENICEREUS GRANDIFLORUS FLOWER (UNII: II877K4UNR) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) PHENOXYETHANOL (UNII: HIE492ZZ3T) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) SODIUM BENZOATE (UNII: OJ245FE5EU) TROPAEOLUM MAJUS FLOWERING TOP (UNII: RGT30824HY) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68577-151-01 1 in 1 CARTON 06/01/2023 1 100 mg in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 06/01/2023 Labeler - COSMAX USA, CORPORATION (010990210) Registrant - COSMAX USA, CORPORATION (010990210) Establishment Name Address ID/FEI Business Operations COSMAX USA. CORPORATION 010990210 manufacture(68577-151)

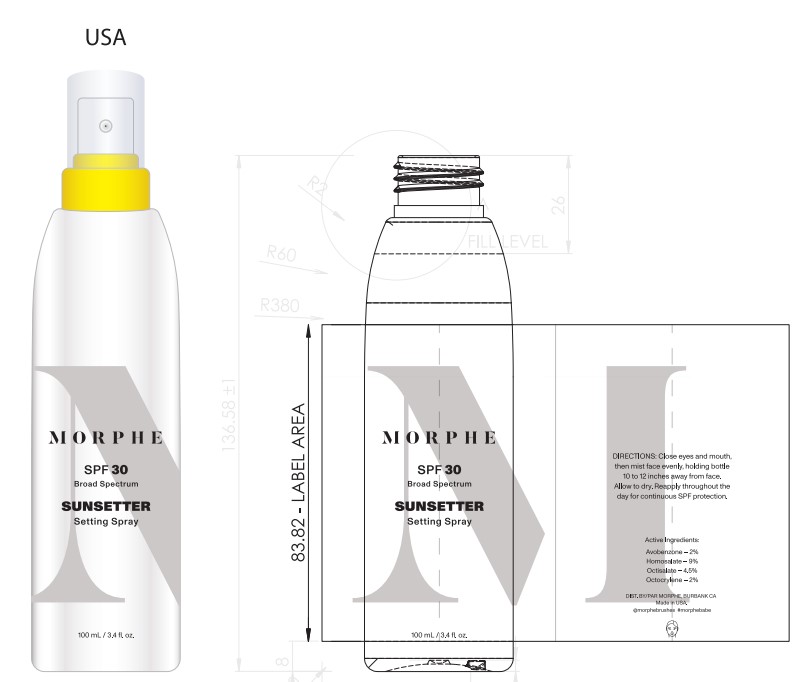

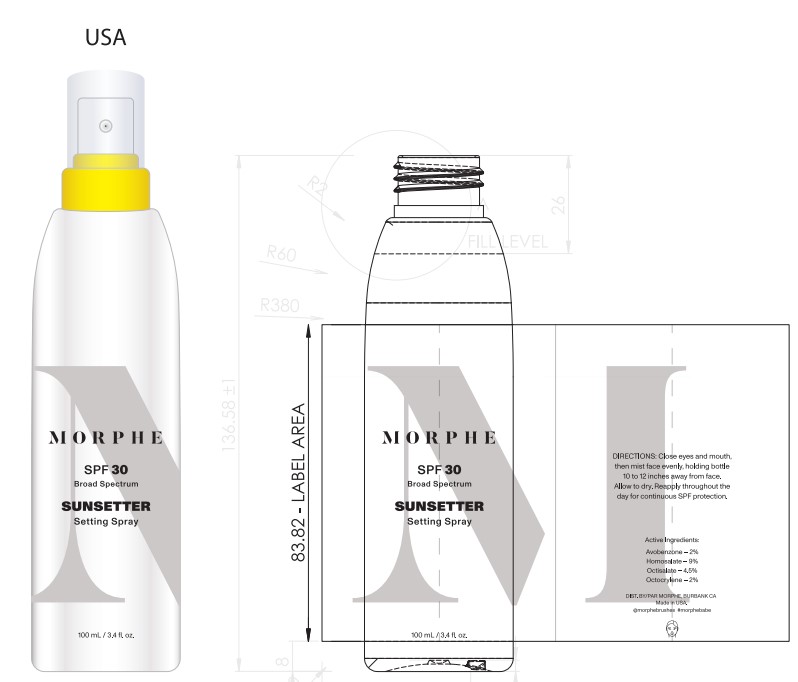 Primary Package(Inner)
Primary Package(Inner)
