Label: NEO-VITAL RX- vitamin a, ascorbic acid, cholecalciferol, dl-alpha tocopheryl acetate, thiamine mononitrate, vitamin b2, niacinamide, pyridoxine hcl, folic acid, cyanocobalamin, calcium carbonate, ferrous fumarate, zinc oxide, cupric oxide, inositol, citrus bioflavonoids fruit complex, boron citrate tablet
- NHRIC Code(s): 50228-276-90
- Packager: ScieGen Pharmaceuticals, Inc
- Category: DIETARY SUPPLEMENT
- DEA Schedule: None
- Marketing Status: Dietary Supplement
Drug Label Information
Updated October 30, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- STATEMENT OF IDENTITY
-
HEALTH CLAIM
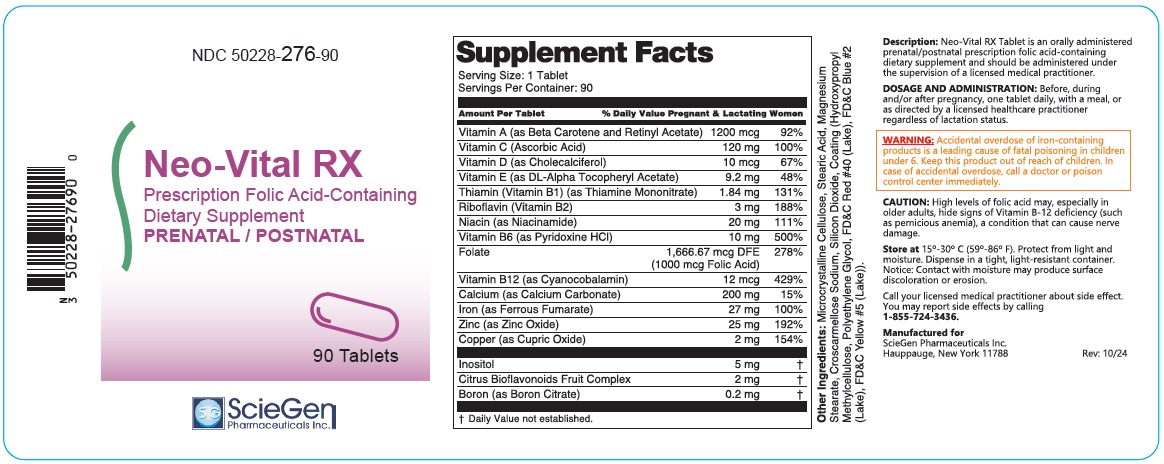
Supplement Facts Serving Size: 1 Tablet Servings Per Container: 90 Amount Per Tablet % Daily Value Pregnant & Lactating Women Vitamin A (as Beta Carotene and Retinyl Acetate) 1200 mcg 92% Vitamin C (Ascorbic Acid) 120 mg 100% Vitamin D (as Cholecalciferol) 10 mcg 67% Vitamin E (as DL-Alpha Tocopheryl Acetate) 9.2 mg 48% Thiamin (Vitamin B1) (as Thiamine Mononitrate) 1.84 mg 131% Riboflavin (Vitamin B2) 3 mg 188% Niacin (as Niacinamide) 20 mg 111% Vitamin B6 (as Pyridoxine HCl) 10 mg 500% Folate 1,666.67 mcg DFE
(1000 mcg Folic Acid)278% Vitamin B12 (as Cyanocobalamin) 12 mcg 429% Calcium (as Calcium Carbonate) 200 mg 15% Iron (as Ferrous Fumarate) 27 mg 100% Zinc (as Zinc Oxide) 25 mg 192% Copper (as Cupric Oxide) 2 mg 154% Inositol 5 mg † Citrus Bioflavonoids Fruit Complex 2 mg † Boron (as Boron Citrate) 0.2 mg † † Daily Value not established. Other Ingredients: Microcrystalline Cellulose, Stearic Acid, Magnesium Stearate, Croscarmellose Sodium, Silicon Dioxide, Coating (Hydroxypropyl Methylcellulose, Polyethylene Glycol, FD&C Red #40 (Lake), FD&C Blue #2 (Lake), FD&C Yellow #5(Lake)).
Description:
Neo-Vital RX Tablet is an orally administered prenatal/postnatal prescription folic acid-containing dietary supplement and should be administered under the supervision of a licensed medical practitioner.
- DOSAGE AND ADMINISTRATION:
- WARNING:
-
CAUTION:
High levels of folic acid may, especially in older adults, hide signs of Vitamin B-12 deficiency (such as pernicious anemia), a condition that can cause nerve damage.
Store at 15°-30° C (59°-86° F). Protect from light and moisture. Dispense in a tight, light-resistant container. Notice: Contact with moisture may produce surface discoloration or erosion.
Call your licensed medical practitioner about side effect. You may report side effects by calling 1-855-724-3436.
Manufactured for
ScieGen Pharmaceuticals Inc.
Hauppauge, New York 11788
Rev. 10/24
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NEO-VITAL RX
vitamin a, ascorbic acid, cholecalciferol, dl-alpha tocopheryl acetate, thiamine mononitrate, vitamin b2, niacinamide, pyridoxine hcl, folic acid, cyanocobalamin, calcium carbonate, ferrous fumarate, zinc oxide, cupric oxide, inositol, citrus bioflavonoids fruit complex, boron citrate tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:50228-276 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VITAMIN A (UNII: 81G40H8B0T) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 1200 ug ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 120 mg VITAMIN D (UNII: 9VU1KI44GP) (CHOLECALCIFEROL - UNII:1C6V77QF41) VITAMIN D 10 ug .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) (.ALPHA.-TOCOPHEROL, DL- - UNII:7QWA1RIO01) .ALPHA.-TOCOPHEROL, DL- 9.2 mg THIAMINE (UNII: X66NSO3N35) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.84 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 3 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 10 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1666.67 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 12 ug CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CATION 200 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 27 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 25 mg CUPRIC OXIDE (UNII: V1XJQ704R4) (CUPRIC CATION - UNII:8CBV67279L) CUPRIC CATION 2 mg INOSITOL (UNII: 4L6452S749) (INOSITOL - UNII:4L6452S749) INOSITOL 5 mg CITRUS BIOFLAVONOIDS (UNII: BD70459I50) (HESPERIDIN - UNII:E750O06Y6O) CITRUS BIOFLAVONOIDS 2 mg BORON CITRATE (UNII: S043P4DV22) (HESPERIDIN - UNII:E750O06Y6O) BORON CITRATE 0.2 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) STEARIC ACID (UNII: 4ELV7Z65AP) MAGNESIUM STEARATE (UNII: 70097M6I30) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) HYDROXYPROPYL METHYLCELLULOSE (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:50228-276-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 10/31/2024 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 6 mm Labeler - ScieGen Pharmaceuticals, Inc (079391286) Establishment Name Address ID/FEI Business Operations ScieGen Pharmaceuticals, Inc 079391286 manufacture(50228-276)