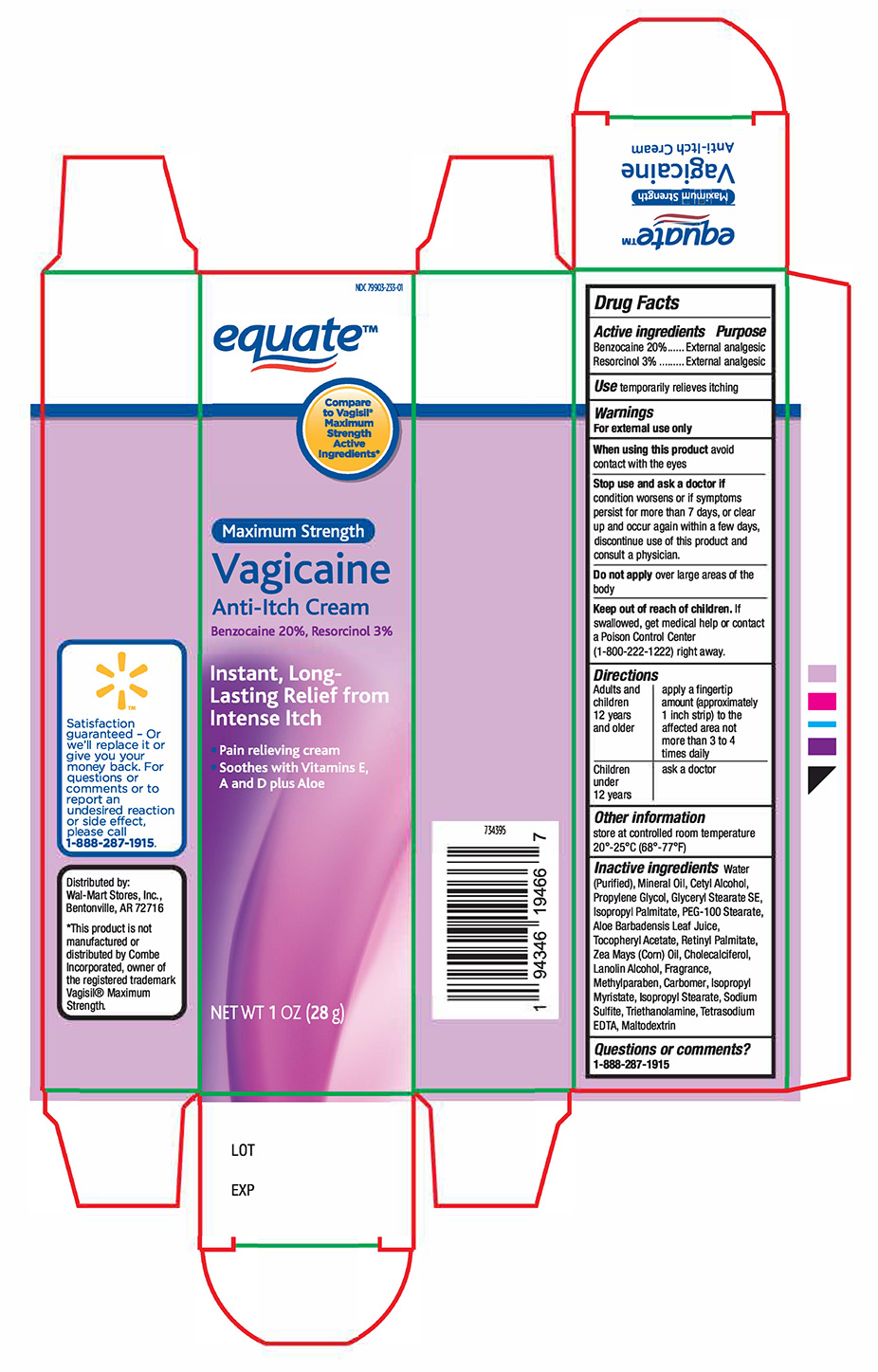
Label: EQUATE VAGICAINE- 20% benzocaine and 3% resorcinol cream cream
- NDC Code(s): 79903-233-01
- Packager: Walmart Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 3, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Purpose
- Uses
- Warnings
- Stop use and ask if doctor if
- Keep out of the reach of children
- Directions
- Other information
-
Inactive ingredients
Water, Mineral Oil, Cetyl Alcohol, Propylene Glycol, Glyceryl Stearate, PEG-100 Stearate, Isopropyl Palmitate, Aloe Barbadensis Leaf Extract, Tocopheryl Acetate, Retinyl Palmitate, Zea Mays (Corn) Oil, Cholecalciferol, Lanolin Alcohol, Fragrance, Methylparaben, Carbomer, Isopropyl Myristate, Isopropyl Stearate, Sodium Sulfite, Triethanolamine, Trisodium EDTA, Maltodextrin
- Principal Dispaly panel Tube and Carton
-
INGREDIENTS AND APPEARANCE
EQUATE VAGICAINE
20% benzocaine and 3% resorcinol cream creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:79903-233 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE HYDROCHLORIDE (UNII: OG625Z9LEO) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE HYDROCHLORIDE 200 mg in 1 g RESORCINOL (UNII: YUL4LO94HK) (RESORCINOL - UNII:YUL4LO94HK) RESORCINOL 30 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MINERAL OIL (UNII: T5L8T28FGP) CETYL ALCOHOL (UNII: 936JST6JCN) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) PEG-100 STEARATE (UNII: YD01N1999R) ALOE VERA LEAF (UNII: ZY81Z83H0X) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) VITAMIN A PALMITATE (UNII: 1D1K0N0VVC) CORN OIL (UNII: 8470G57WFM) CHOLECALCIFEROL (UNII: 1C6V77QF41) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) METHYLPARABEN (UNII: A2I8C7HI9T) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) ISOPROPYL STEARATE (UNII: 43253ZW1MZ) SODIUM SULFITE (UNII: VTK01UQK3G) TROLAMINE (UNII: 9O3K93S3TK) TETRASODIUM EDETATE DIHYDRATE (UNII: 3JGX4KKZ4A) MALTODEXTRIN (UNII: 7CVR7L4A2D) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:79903-233-01 1 in 1 CARTON 12/01/2023 1 28 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 12/01/2023 Labeler - Walmart Inc. (051957769)