Label: KNEE JOINT PATCH- panax notoginseng root patch
- NDC Code(s): 52784-022-01
- Packager: ZHENGZHOU GIANT BIOCHEMISTRY GROUP CO.,LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
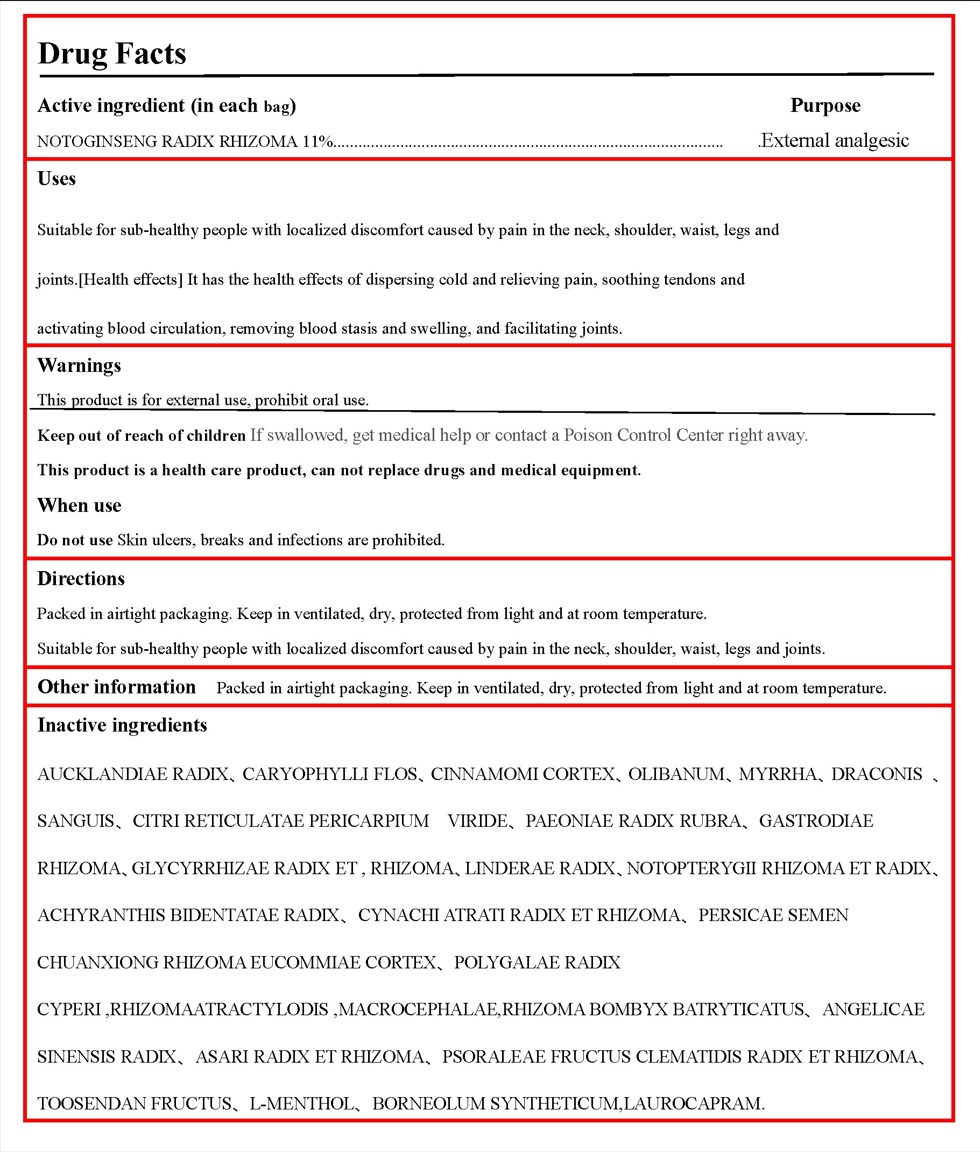
- Active ingredient
- Purpose
-
Use
Suitable for sub-healthy people with localized discomfort caused by pain in the neck, shoulder, waist, legs and
joints.[Health effects] It has the health effects of dispersing cold and relieving pain, soothing tendons and
activating blood circulation, removing blood stasis and swelling, and facilitating joints.
-
Warnings
This product is for external use, prohibit oral use.
When using this product
A small number of people have a mild local itching sensation during the use of the product, which does not affect the continued use of the product and can gradually disappear after stopping the use of the product.
- Other information
- Directions
-
Inactive ingredients
AUCKLANDIAE RADIX、CARYOPHYLLI FLOS、CINNAMOMI CORTEX、OLIBANUM、MYRRHA、DRACONIS 、SANGUIS、CITRI RETICULATAE PERICARPIUM VIRIDE、PAEONIAE RADIX RUBRA、GASTRODIAE RHIZOMA、GLYCYRRHIZAE RADIX ET , RHIZOMA、LINDERAE RADIX、NOTOPTERYGII RHIZOMA ET RADIX、ACHYRANTHIS BIDENTATAE RADIX、CYNACHI ATRATI RADIX ET RHIZOMA、PERSICAE SEMEN CHUANXIONG RHIZOMA EUCOMMIAE CORTEX、POLYGALAE RADIX CYPERI ,RHIZOMAATRACTYLODIS ,MACROCEPHALAE,RHIZOMA BOMBYX BATRYTICATUS、ANGELICAE SINENSIS RADIX、ASARI RADIX ET RHIZOMA、PSORALEAE FRUCTUS CLEMATIDIS RADIX ET RHIZOMA、TOOSENDAN FRUCTUS、L-MENTHOL、BORNEOLUM SYNTHETICUM,LAUROCAPRAM.
- Label
-
INGREDIENTS AND APPEARANCE
KNEE JOINT PATCH
panax notoginseng root patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:52784-022 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PANAX NOTOGINSENG ROOT (UNII: GQX1C1175U) (PANAX NOTOGINSENG ROOT - UNII:GQX1C1175U) PANAX NOTOGINSENG ROOT 11 g in 100 g Inactive Ingredients Ingredient Name Strength NOTOPTERYGIUM INCISUM ROOT (UNII: 5Z2WW4J6RI) ACHYRANTHES BIDENTATA ROOT (UNII: 5QIU26R6P1) MELIA AZEDARACH FRUIT (UNII: 50J4V3X4H8) DRAGON'S BLOOD (UNII: M3YJ2C28IC) LICORICE (UNII: 61ZBX54883) LINDERA AGGREGATA ROOT (UNII: 517Q7XRT2T) CHINESE CINNAMON (UNII: WS4CQ062KM) MYRRH (UNII: JC71GJ1F3L) EUCOMMIA ULMOIDES BARK (UNII: L878N1L0AR) POLYGALA TENUIFOLIA ROOT (UNII: 5S7W573MTU) CYPERUS ROTUNDUS ROOT (UNII: 4B51SRR959) BOMBYX MORI LARVA (UNII: 1WYM0QWX33) MENTHOL (UNII: L7T10EIP3A) CLOVE (UNII: K48IKT5321) FRANKINCENSE (UNII: R9XLF1R1WM) PAEONIA VEITCHII ROOT (UNII: VX6GD6M93V) ANGELICA SINENSIS ROOT (UNII: B66F4574UG) CULLEN CORYLIFOLIUM FRUIT (UNII: 78AD6Z52S6) DOLOMIAEA COSTUS ROOT (UNII: RUP970CGR9) ANGELICA DAHURICA ROOT (UNII: 1V63N2S972) PRUNUS PERSICA SEED (UNII: V9C81470RR) ASARUM HETEROTROPOIDES VAR. MANDSHURICUM ROOT (UNII: HK49KSK8L3) BORNEOL (UNII: M89NIB437X) VINCETOXICUM ATRATUM ROOT (UNII: 7DQZ24B35Y) LIGUSTICUM SINENSE SUBSP. CHUANXIONG ROOT (UNII: RR83T99U97) TANGERINE PEEL, UNRIPE (UNII: B077C3NLJV) CLEMATIS CHINENSIS ROOT (UNII: 8Z18N528CU) GASTRODIA ELATA TUBER (UNII: 08F85I5YAV) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:52784-022-01 0.55 g in 1 PATCH; Type 0: Not a Combination Product 12/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/18/2023 Labeler - ZHENGZHOU GIANT BIOCHEMISTRY GROUP CO.,LTD (527840360) Establishment Name Address ID/FEI Business Operations ZHENGZHOU GIANT BIOCHEMISTRY GROUP CO.,LTD 527840360 manufacture(52784-022)