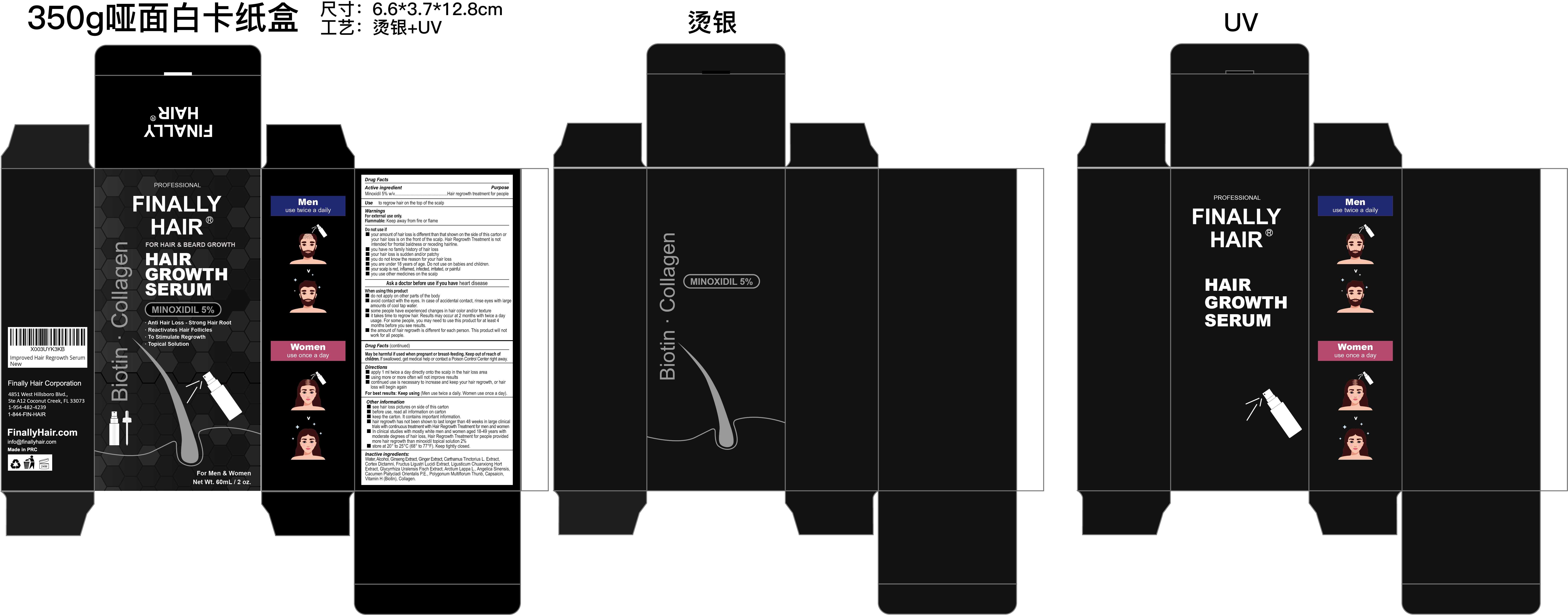
Label: FINALLY HAIR MINOXIDIL 5% HAIR GROWTH SERUM- minoxidil 5% hair growth serum liquid
- NDC Code(s): 81653-012-01
- Packager: Guangzhou Jianyuan Biological Technology Co.,Ltd
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated December 19, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
-
Do not use
■your amount of hair loss is different than that shown on the side of this carton or your hair loss is on the front of the scalp. Hair Regrowth Treatment is not intended for frontal baldness or receding hairline.
■ you have no family history of hair loss
■ your hair loss is sudden and/or patchy
■ you do not know the reason for your hair loss
■ you are under 18 years of age. Do not use on babies and children.
■ your scalp is red, inflamed, infected, irritated, or painful
■ you use other medicines on the scalp -
When Using
■ do not apply on other parts of the body
■ avoid contact with the eyes. In case of accidental contact, rinse eyes with large amounts of cool tap water.
■ some people have experienced changes in hair color and/or texture
■ it takes time to regrow hair. Results may occur at 2 months with twice a day usage. For some people, you may need to use this product for at least 4 months before you see results.
■ the amount of hair regrowth is different for each person. This product will not work for all people. - Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
- Directions
-
Other information
■ see hair loss pictures on side of this carton
■ before use, read all information on carton
■ keep the carton. It contains important information.
■ hair regrowth has not been shown to last longer than 48 weeks in large clinical
trials with continuous treatment with Hair Regrowth Treatment for men and women
■ In clinical studies with mostly white men and women aged 18-49 years with
moderate degrees of hair loss, Hair Regrowth Treatment for people provided
more hair regrowth than minoxidil topical solution 2%
■ store at 20° to 25°C (68° to 77°F). Keep tightly closed. -
Inactive ingredients
Water, Alcohol, Ginseng Extract, Ginger Extract, Carthamus Tinctorius L. Extract,
Cortex Dictamni, Fructus Ligustri Lucidi Extract, Ligusticum Chuanxiong Hort
Extract, Glycyrrhiza Uralensis Fisch Extract, Arctium Lappa L., Angelica Sinensis,
Cacumen Platycladi Orientalis P.E., Polygonum Multiflorum Thunb, Capsaicin,
Vitamin H (Biotin), Collagen. - Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FINALLY HAIR MINOXIDIL 5% HAIR GROWTH SERUM
minoxidil 5% hair growth serum liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:81653-012 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MINOXIDIL (UNII: 5965120SH1) (MINOXIDIL - UNII:5965120SH1) MINOXIDIL 5 g in 100 mL Inactive Ingredients Ingredient Name Strength ANGELICA SINENSIS ROOT (UNII: B66F4574UG) BOK-CHOY (UNII: EKJ8Z7DEWI) ARCTIUM LAPPA WHOLE (UNII: 73070DU1LA) REYNOUTRIA MULTIFLORA ROOT OIL (UNII: 1HIE915O2J) CARTHAMUS TINCTORIUS WHOLE (UNII: 5EMV416J82) RUBUS CHINGII WHOLE (UNII: A32A8VI76N) ASIAN GINSENG (UNII: CUQ3A77YXI) GINGER (UNII: C5529G5JPQ) DICTAMNUS DASYCARPUS ROOT (UNII: 6153LEN214) CAPSAICIN (UNII: S07O44R1ZM) COLLAGEN ALPHA-1(II) CHAIN (UNII: 86V2W84PW4) BIOTIN (UNII: 6SO6U10H04) LIGUSTICUM SINENSE SUBSP. CHUANXIONG ROOT (UNII: RR83T99U97) GLYCYRRHIZA URALENSIS WHOLE (UNII: 8XW1DS8UIR) WATER (UNII: 059QF0KO0R) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81653-012-01 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075357 12/18/2023 Labeler - Guangzhou Jianyuan Biological Technology Co.,Ltd (548120189) Establishment Name Address ID/FEI Business Operations Guangzhou Jianyuan Biological Technology Co.,Ltd 548120189 manufacture(81653-012)