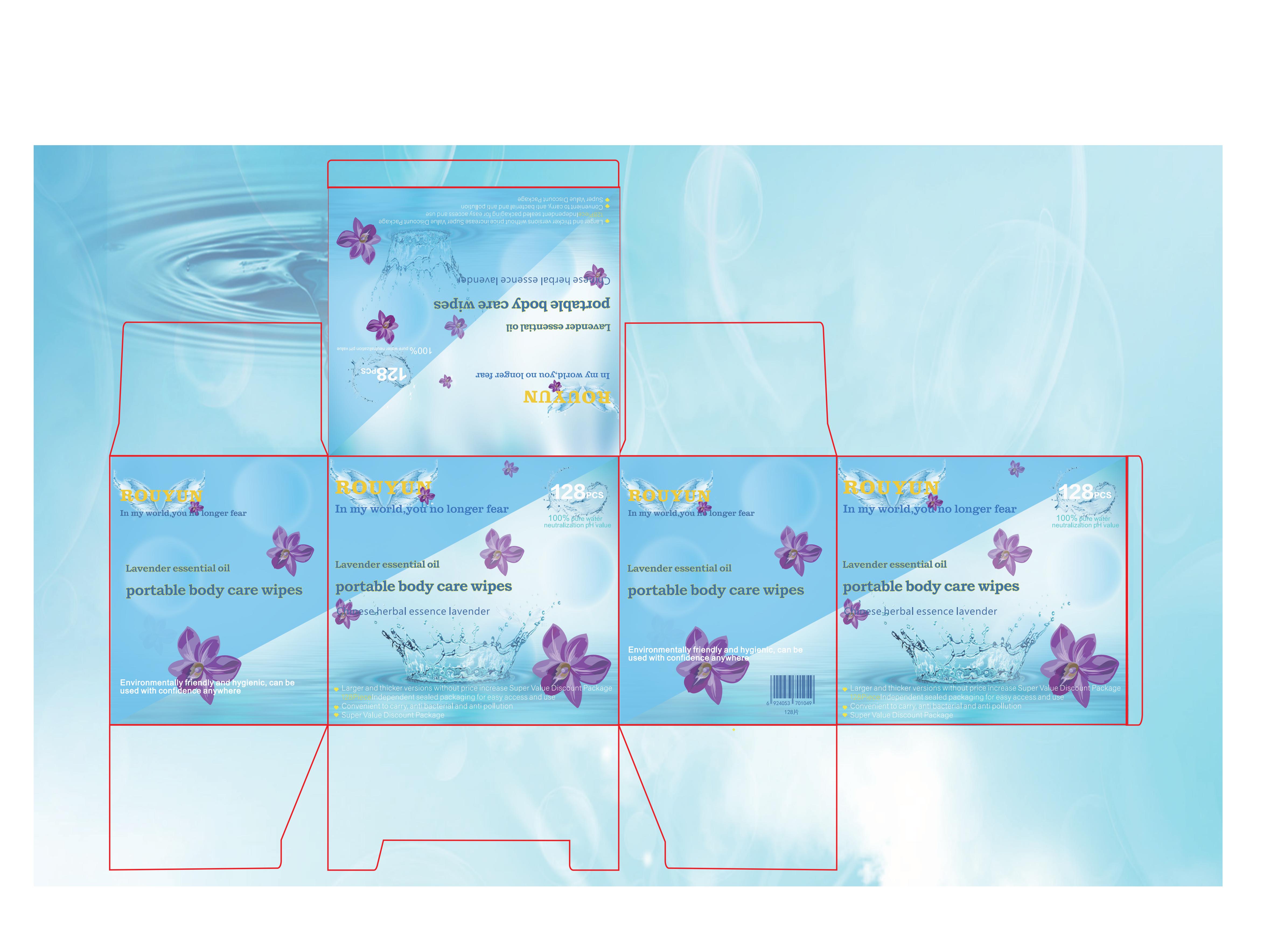
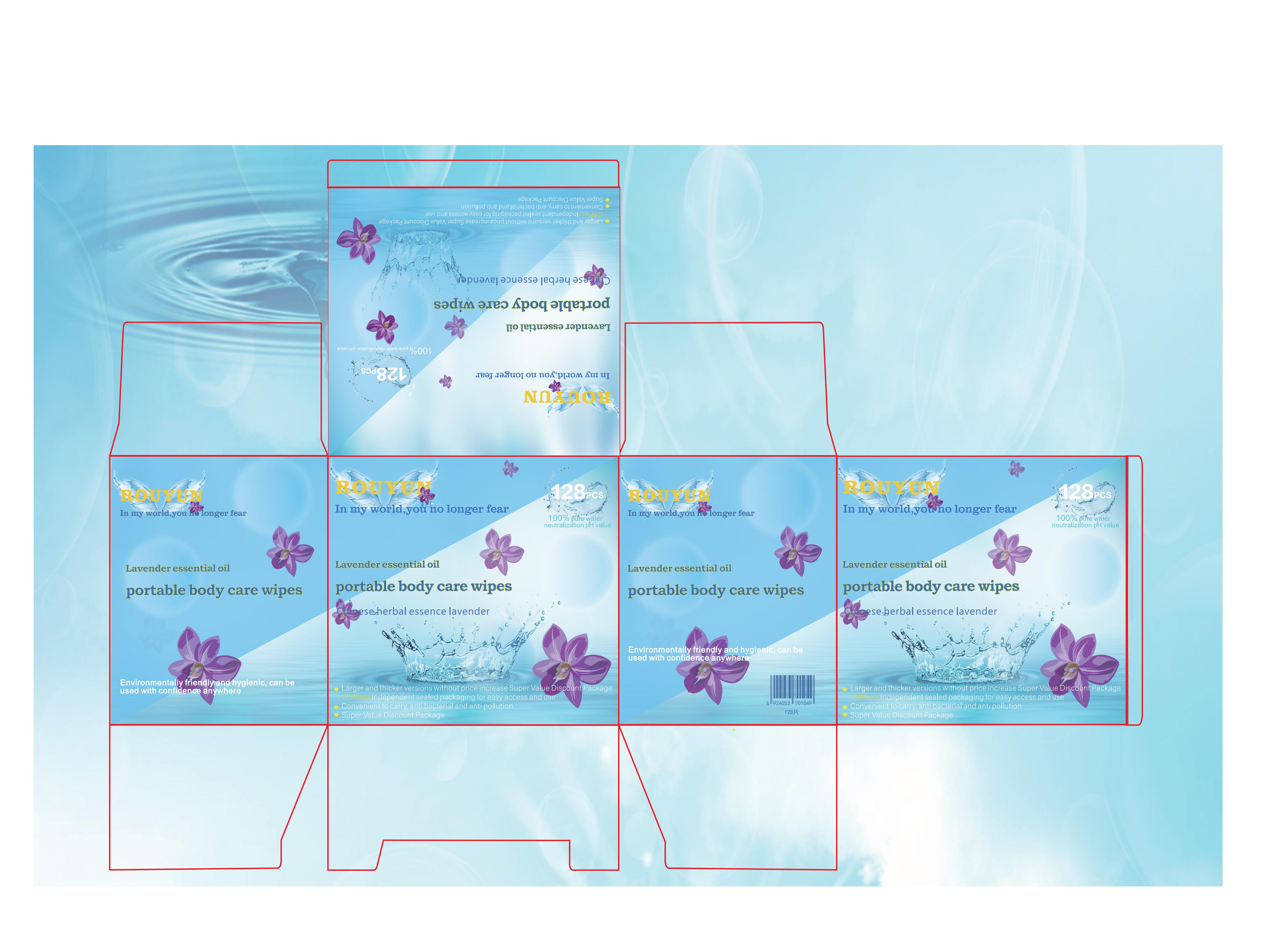
Label: ROUYUN LAVENDER ESSENTIAL OIL PORTABLE BODY CARE WIPES cloth
-
NDC Code(s):
43116-033-01,
43116-033-02,
43116-033-03,
43116-033-04, view more43116-033-05, 43116-033-06, 43116-033-07, 43116-033-08
- Packager: Shenzhen Shierjie Biological Engineering Co., LTD
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Use
- Warnings
- Do not use
- When Using
- Stop Use
- Ask Doctor
- Keep Oot Of Reach Of Children
-
Directions
Tear open the packaging gap, take out the wet wipes, lay them flat and open them to wipe the skin without washing with water.
Daily skin and sweat wiping, suitable for any skin color and type, suitable for both men and women of all ages.
Unable to prevent the spread of sexually transmitted diseases. - Other information
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ROUYUN LAVENDER ESSENTIAL OIL PORTABLE BODY CARE WIPES
rouyun lavender essential oil portable body care wipes clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43116-033 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.09 g in 100 Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43116-033-01 1 in 1 BAG; Type 0: Not a Combination Product 12/17/2023 2 NDC:43116-033-02 10 in 1 BOX; Type 0: Not a Combination Product 12/17/2023 3 NDC:43116-033-03 16 in 1 BOX; Type 0: Not a Combination Product 12/17/2023 4 NDC:43116-033-04 50 in 1 BOX; Type 0: Not a Combination Product 12/17/2023 5 NDC:43116-033-05 64 in 1 BOX; Type 0: Not a Combination Product 12/17/2023 6 NDC:43116-033-06 80 in 1 BOX; Type 0: Not a Combination Product 12/17/2023 7 NDC:43116-033-07 100 in 1 BOX; Type 0: Not a Combination Product 12/17/2023 8 NDC:43116-033-08 128 in 1 BOX; Type 0: Not a Combination Product 12/17/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M005 12/17/2023 Labeler - Shenzhen Shierjie Biological Engineering Co., LTD (547610261) Establishment Name Address ID/FEI Business Operations Shenzhen Shierjie Biological Engineering Co., LTD 547610261 label(43116-033) , manufacture(43116-033)