Label: VOQUEZNA- vonoprazan fumarate tablet
- NDC Code(s): 81520-100-30, 81520-200-30
- Packager: Phathom Pharmaceuticals Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: New Drug Application
Drug Label Information
Updated November 18, 2025
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VOQUEZNA® safely and effectively. See full prescribing information for VOQUEZNA.
VOQUEZNA (vonoprazan) tablets, for oral use
Initial U.S. Approval: 2022INDICATIONS AND USAGE
VOQUEZNA is a potassium-competitive acid blocker indicated:
- for healing of all grades of erosive esophagitis and relief of heartburn associated with erosive esophagitis in adults. (1)
- to maintain healing of all grades of erosive esophagitis and relief of heartburn associated with erosive esophagitis in adults. (1)
- for the relief of heartburn associated with non-erosive gastroesophageal reflux disease in adults. (1)
- in combination with amoxicillin and clarithromycin for the treatment of Helicobacter pylori (H. pylori) infection in adults. (1)
- in combination with amoxicillin for the treatment of H. pylori infection in adults. (1)
DOSAGE AND ADMINISTRATION
Recommended Dosage:
- Healing of Erosive Esophagitis: 20 mg once daily for 8 weeks. (2.1)
- Maintenance of Healed Erosive Esophagitis: 10 mg once daily for up to 6 months. (2.1)
- Relief of Heartburn Associated with Non-Erosive Gastroesophageal Reflux Disease: 10 mg once daily for 4 weeks. (2.1)
- Treatment of H. pylori Infection: see full prescribing information. (2.1)
- See also full prescribing information for the recommended dosage by indication for patients with renal or hepatic impairment. (2.2, 2.3)
Administration Instructions:
DOSAGE FORMS AND STRENGTHS
Tablets: 10 mg and 20 mg of vonoprazan. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Gastric Malignancy: Symptomatic response to treatment does not preclude the presence of gastric malignancy; consider additional follow-up and diagnostic testing. (5.1)
- Acute Tubulointerstitial Nephritis: Discontinue treatment and evaluate patients. (5.2)
- Clostridioides difficile-Associated Diarrhea (CDAD): May be associated with an increased risk; use the shortest duration of treatment appropriate to the condition. (5.3)
- Bone Fracture, including Osteoporosis-related Fracture: Use the shortest duration of treatment appropriate to the condition. (5.4)
- Severe Cutaneous Adverse Reactions: Discontinue at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation. (5.5)
- Vitamin B12 (Cobalamin) Deficiency: Long-term use may lead to malabsorption or deficiency; consider further workup if clinical symptoms are present. (5.6)
- Hypomagnesemia and Mineral Metabolism: Hypomagnesemia may lead to hypocalcemia and/or hypokalemia. Consider monitoring magnesium and calcium levels in at-risk patients, or if there is concomitant use of digoxin or other drugs that cause hypomagnesemia. (5.7)
- Interactions with Investigations for Neuroendocrine Tumors: Increased chromogranin A (CgA) levels may interfere with diagnostic investigations; temporarily stop VOQUEZNA at least 4 weeks before assessing CgA levels. (5.8, 7)
- Fundic Gland Polyps: Risk increases with long-term use; use the shortest duration of treatment appropriate to the condition. (5.9)
ADVERSE REACTIONS
Most common adverse reactions in VOQUEZNA-treated patients are:
- Healing of Erosive Esophagitis (≥2%): gastritis, diarrhea, abdominal distension, abdominal pain, and nausea. (6.1)
- Maintenance of Healed Erosive Esophagitis (≥3%): gastritis, abdominal pain, dyspepsia, hypertension, and urinary tract infection. (6.1)
- Relief of Heartburn Associated with Non-Erosive Gastroesophageal Reflux Disease (≥2%): abdominal pain, constipation, diarrhea, nausea, and urinary tract infection. (6.1)
- Treatment of H. pylori Infection (≥2%): diarrhea, dysgeusia, vulvovaginal candidiasis, abdominal pain, headache, hypertension, and nasopharyngitis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Phathom Pharmaceuticals, Inc. at toll-free phone 1-888-775-7428 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See full prescribing information for a list of clinically important drug interactions. (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Recommended Dosage in Patients with Renal Impairment
2.3 Recommended Dosage in Patients with Hepatic Impairment
2.4 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Presence of Gastric Malignancy
5.2 Acute Tubulointerstitial Nephritis
5.3 Clostridioides difficile-Associated Diarrhea
5.4 Bone Fracture
5.5 Severe Cutaneous Adverse Reactions
5.6 Vitamin B12 (Cobalamin) Deficiency
5.7 Hypomagnesemia and Mineral Metabolism
5.8 Interactions with Diagnostic Investigations for Neuroendocrine Tumors
5.9 Fundic Gland Polyps
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Healing of Erosive Esophagitis and Relief of Heartburn
14.2 Maintenance of Healed Erosive Esophagitis and Relief of Heartburn
14.3 Relief of Heartburn Associated with Non-Erosive Gastroesophageal Reflux Disease
14.4 Treatment of Helicobacter pylori Infection
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
VOQUEZNA is indicated:
- for healing of all grades of erosive esophagitis and relief of heartburn associated with erosive esophagitis in adults.
- to maintain healing of all grades of erosive esophagitis and relief of heartburn associated with erosive esophagitis in adults.
- for the relief of heartburn associated with non-erosive gastroesophageal reflux disease in adults.
- in combination with amoxicillin and clarithromycin for the treatment of Helicobacter pylori (H. pylori) infection in adults.
- in combination with amoxicillin for the treatment of H. pylori infection in adults.
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Healing of Erosive Esophagitis
- The recommended adult oral dosage is VOQUEZNA 20 mg once daily for 8 weeks for the treatment of healing of erosive esophagitis and relief of associated heartburn.
Maintenance of Healed Erosive Esophagitis
- The recommended adult oral dosage is VOQUEZNA 10 mg once daily for up to 6 months for the maintenance of healed erosive esophagitis and relief of associated heartburn.
Relief of Heartburn Associated with Non-Erosive Gastroesophageal Reflux Disease
- The recommended adult oral dosage is VOQUEZNA 10 mg once daily for 4 weeks.
Treatment of H. pylori Infection
- Triple Therapy: The recommended adult oral dosage is VOQUEZNA 20 mg plus amoxicillin 1,000 mg plus clarithromycin 500 mg, each given twice daily (in the morning and evening, 12 hours apart) for 14 days.
- Dual Therapy: The recommended adult oral dose is VOQUEZNA 20 mg given twice daily (in the morning and evening) plus amoxicillin 1,000 mg three times daily (in the morning, mid-day, and evening) for 14 days.
- Also refer to the amoxicillin and clarithromycin full prescribing information.
2.2 Recommended Dosage in Patients with Renal Impairment
Healing of Erosive Esophagitis
The recommended dosage of VOQUEZNA in adult patients with renal impairment is described in Table 1 below [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Table 1: Recommended VOQUEZNA Dosage in Patients with Renal Impairment: Healing of Erosive Esophagitis Estimated glomerular filtration rate (GFR) Recommended Dosage 30 mL/minute or greater 20 mg once daily Less than 30 mL/minute 10 mg once daily Maintenance of Healed Erosive Esophagitis or Relief of Heartburn Associated with Non-Erosive Gastroesophageal Reflux Disease
The recommended dosage of VOQUEZNA in adult patients with renal impairment is the same as for adult patients with normal renal function [see Dosage and Administration (2.1)].
Treatment of H. pylori Infection
The recommended dosage of VOQUEZNA in adult patients with renal impairment is described in Table 2 below [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
Table 2: Recommended VOQUEZNA Dosage in Patients with Renal Impairment: Treatment of H. pylori Infection* Estimated GFR Recommended Dosage - *
- Also refer to the Dosage and Administration section of the amoxicillin and clarithromycin prescribing information for dosage recommendations in patients with renal impairment.
30 mL/minute or greater 20 mg twice daily Less than 30 mL/minute Use is not recommended 2.3 Recommended Dosage in Patients with Hepatic Impairment
Healing of Erosive Esophagitis
The recommended dosage of VOQUEZNA in adult patients with hepatic impairment is described in Table 3 below [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
Table 3: Recommended VOQUEZNA Dosage in Patients with Hepatic Impairment: Healing of Erosive Esophagitis Classification Recommended Dosage Child-Pugh Class A 20 mg once daily Child-Pugh Class B 10 mg once daily Child-Pugh Class C 10 mg once daily Maintenance of Healed Erosive Esophagitis or Relief of Heartburn Associated with Non-Erosive Gastroesophageal Reflux Disease
The recommended dosage of VOQUEZNA in adult patients with hepatic impairment is the same as for patients with normal hepatic function [see Dosage and Administration (2.1)].
Treatment of H. pylori Infection
The recommended dosage of VOQUEZNA in adult patients with hepatic impairment is described in Table 4 below [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
Table 4: Recommended VOQUEZNA Dosage in Patients with Hepatic Impairment: Treatment of H. pylori Infection Classification Recommended Dosage Child-Pugh Class A 20 mg twice daily Child-Pugh Class B Use is not recommended Child-Pugh Class C Use is not recommended 2.4 Administration Instructions
- Take VOQUEZNA with or without food [see Clinical Pharmacology (12.3)].
- Swallow VOQUEZNA tablets whole; do not chew or crush the tablet.
- Missed doses:
- For the healing or maintenance of healed erosive esophagitis, or the relief of heartburn associated with non-erosive gastroesophageal reflux disease: If a dose is missed, administer VOQUEZNA as soon as possible within 12 hours after the missed dose. If more than 12 hours have passed, skip the missed dose and administer the next dose at the regularly scheduled time.
- For the treatment of H. pylori infection: If a dose is missed, administer VOQUEZNA as soon as possible within 4 hours after the missed dose. If more than 4 hours have passed, skip the missed dose and administer the next dose at the regularly scheduled time. Continue the normal dosing schedule until the treatment is completed.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
- VOQUEZNA is contraindicated in patients with a known hypersensitivity to vonoprazan or any component of VOQUEZNA. Reactions have included anaphylactic shock [see Adverse Reactions (6.2) and Description (11)].
- VOQUEZNA is contraindicated with rilpivirine-containing products [see Drug Interactions (7)].
- For information about contraindications of antibacterial agents (clarithromycin and amoxicillin) indicated in combination with VOQUEZNA, refer to the Contraindications section of the corresponding prescribing information.
-
5 WARNINGS AND PRECAUTIONS
5.1 Presence of Gastric Malignancy
In adults, symptomatic response to therapy with VOQUEZNA does not preclude the presence of gastric malignancy. Consider additional follow-up and diagnostic testing in patients who have a suboptimal response or an early symptomatic relapse after completing treatment with VOQUEZNA. In older patients, also consider endoscopy.
5.2 Acute Tubulointerstitial Nephritis
Acute tubulointerstitial nephritis (TIN) has been reported with VOQUEZNA [see Adverse Reactions (6.1)]. If suspected, discontinue VOQUEZNA and evaluate patients with suspected acute TIN.
5.3 Clostridioides difficile-Associated Diarrhea
Published observational studies suggest that proton pump inhibitors (PPIs) may be associated with an increased risk of Clostridioides difficile-associated diarrhea (CDAD), especially in hospitalized patients. VOQUEZNA, another drug that blocks the proton pump to inhibit gastric acid production, may also increase the risk of CDAD. Consider CDAD in patients with diarrhea that does not improve [see Adverse Reactions (6.2)]. Use the shortest duration of VOQUEZNA appropriate to the condition being treated.
CDAD has been reported with use of nearly all antibacterial agents. For more information specific to antibacterial agents (clarithromycin and amoxicillin) indicated for use in combination with VOQUEZNA, refer to the Warnings and Precautions section of the corresponding prescribing information.
5.4 Bone Fracture
Several published observational studies suggest that PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist, or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term therapy (a year or longer). Bone fracture, including osteoporosis-related fracture, has also been reported with vonoprazan. Use the shortest duration of VOQUEZNA appropriate to the condition being treated [see Dosage and Administration (2.1)]. Patients at risk for osteoporosis-related fractures should be managed according to the established treatment guidelines.
5.5 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) have been reported with VOQUEZNA [see Adverse Reactions (6.2)].
Discontinue VOQUEZNA at the first signs or symptoms of severe cutaneous adverse reactions or other signs of hypersensitivity and consider further evaluation.
5.6 Vitamin B12 (Cobalamin) Deficiency
Long-term use of acid-suppressing drugs can lead to malabsorption of Vitamin B12 caused by hypo- or achlorhydria. Vitamin B12 deficiency has been reported postmarketing with vonoprazan [see Adverse Reactions (6.2)]. If clinical symptoms consistent with Vitamin B12 deficiency are observed in patients treated with VOQUEZNA consider further workup.
5.7 Hypomagnesemia and Mineral Metabolism
Hypomagnesemia has been reported postmarketing with vonoprazan [see Adverse Reactions (6.2)]. Hypomagnesemia may lead to hypocalcemia and/or hypokalemia and may exacerbate underlying hypocalcemia in at-risk patients.
Consider monitoring magnesium levels prior to initiation of VOQUEZNA and periodically in patients expected to be on prolonged treatment, in patients taking drugs that may have increased toxicity in the presence of hypomagnesemia (e.g., digoxin), or drugs that may cause hypomagnesemia (e.g., diuretics). Treatment of hypomagnesemia may require magnesium replacement and discontinuation of VOQUEZNA.
Consider monitoring magnesium and calcium levels prior to initiation of VOQUEZNA and periodically while on treatment in patients with a preexisting risk of hypocalcemia (e.g., hypoparathyroidism). Supplement with magnesium and/or calcium, as necessary. If hypocalcemia is refractory to treatment, consider discontinuing VOQUEZNA.
5.8 Interactions with Diagnostic Investigations for Neuroendocrine Tumors
Serum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors. Temporarily discontinue VOQUEZNA treatment at least 4 weeks before assessing CgA levels and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g., for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary [see Drug Interactions (7) and Clinical Pharmacology (12.2)].
5.9 Fundic Gland Polyps
Use of VOQUEZNA is associated with a risk of fundic gland polyps that increases with long-term use, especially beyond one year. Fundic gland polyps have been reported with vonoprazan in clinical trials and postmarketing use with PPIs. Most patients who developed fundic gland polyps were asymptomatic and fundic gland polyps were identified incidentally on endoscopy. Use the shortest duration of VOQUEZNA appropriate to the condition being treated [see Dosage and Administration (2.1)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in labeling:
- Acute Tubulointerstitial Nephritis [see Warnings and Precautions (5.2)]
- Clostridioides difficile-Associated Diarrhea [see Warnings and Precautions (5.3)]
- Bone Fracture [see Warnings and Precautions (5.4)]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.5)]
- Vitamin B12 (Cobalamin) Deficiency [see Warnings and Precautions (5.6)]
- Hypomagnesemia and Mineral Metabolism [see Warnings and Precautions (5.7)]
- Fundic Gland Polyps [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Healing of Erosive Esophagitis and Maintenance of Healed Erosive Esophagitis
The safety of VOQUEZNA was evaluated in a randomized, active-controlled, double-blind two phase trial for the healing of erosive esophagitis (2 to 8 weeks) and maintenance of healed erosive esophagitis (through 24 weeks) conducted in the United States and Europe [see Clinical Studies (14.1), (14.2)].
Adverse reactions reported in at least 2% of patients in the VOQUEZNA 20 mg once daily arm in the healing phase are presented in Table 5.
Table 5: Adverse Reactions* in a Clinical Trial of Adult Patients with All Grades of Erosive Esophagitis† (2 to 8 Week Healing Phase) Adverse Reactions VOQUEZNA
20 mg Once Daily
N=514
%Lansoprazole
30 mg Once Daily
N=510
%Gastritis‡ 3 2 Diarrhea‡ 2 3 Abdominal distension 2 1 Abdominal pain‡ 2 1 Nausea 2 1 Adverse reactions reported in at least 3% of patients in the VOQUEZNA 10 mg once daily arm of the maintenance phase are shown in Table 6.
Table 6: Adverse Reactions* in a Clinical Trial of Adult Patients with All Grades of Erosive Esophagitis† (24 Week Maintenance Phase) Adverse Reactions VOQUEZNA
10 mg Once Daily
N=296
%Lansoprazole
15 mg Once Daily
N=297
%Gastritis‡ 6 3 Abdominal pain‡ 4 2 Dyspepsia 4 3 Hypertension‡ 3 2 Urinary tract infection 3 2 COVID-19
COVID-19 was reported in the healing phase in 11 (2%) VOQUEZNA-treated patients and 9 (2%) lansoprazole-treated patients, and in the maintenance phase in 18 (6%) VOQUEZNA-treated patients and 20 (7%) lansoprazole-treated patients.
Other Clinical Trials of Erosive Esophagitis
Adverse reactions reported in the United States trial were similar to those reported in 4 additional randomized, active-controlled, double-blind studies of vonoprazan compared to lansoprazole conducted outside of the United States (two 8-week trials of healing of erosive esophagitis and two 24-week maintenance of healed erosive esophagitis trials).
Relief of Heartburn Associated with Non-Erosive Gastroesophageal Reflux Disease
The safety of VOQUEZNA 10 mg once daily for the relief of heartburn associated with non-erosive gastroesophageal reflux disease was evaluated in a randomized, placebo-controlled, double-blind, four-week trial with a 20-week extension phase conducted in the United States [see Clinical Studies (14.3)]. Patients initially randomized to placebo in the 4-week placebo-controlled phase were re-randomized to VOQUEZNA 10 mg once daily or a higher dosage of VOQUEZNA for 20 weeks in the extension phase.
Adverse reactions reported in at least 2% of patients in the VOQUEZNA 10 mg once daily arm in the 4-week placebo-controlled phase are presented in Table 7.
Table 7: Adverse Reactions* in a Clinical Trial of Adult Patients with Non-Erosive Gastroesophageal Reflux Disease (4-week Placebo-Controlled Phase) Adverse Reactions VOQUEZNA 10 mg Once Daily
N=259
%Placebo Once Daily
N=256
%Abdominal pain† 2 2 Constipation 2 1 Diarrhea 2 1 Nausea 2 <1 Urinary tract infection 2 1 Other adverse reactions: Upper respiratory tract infection (4%) and sinusitis (3%) were reported in patients who received VOQUEZNA 10 mg once daily in the 20-week extension phase.
COVID-19
COVID-19 was reported in the 4-week placebo-controlled phase in 3 (1%) patients who received VOQUEZNA 10 mg once daily and 3 (1%) patients who received placebo, and in 24 (7%) patients who received VOQUEZNA 10 mg once daily in the 20-week extension phase.
Less Common Adverse Reactions
Adverse reactions reported in 1% or less of VOQUEZNA-treated patients for the healing or maintenance of healed erosive esophagitis or for the relief of heartburn associated with non-erosive gastroesophageal reflux disease in the United States trials are:
Blood and lymphatic system disorders: anemia, lymphocytosis
Cardiac disorders: tachycardia
Ear and labyrinth disorders: vertigo
Gastrointestinal disorders: duodenal polyp, dry mouth, dysphagia, eructation, flatulence, gastric polyps, vomiting
General disorders and administrative site conditions: asthenia, peripheral edema
Investigations: increased liver enzymes
Metabolism and nutritional disorders: diabetes mellitus
Musculoskeletal system: bone fracture
Nervous system disorders: dizziness, headache, syncope
Psychiatric disorders: depression, insomnia
Renal and urinary disorders: tubulointerstitial nephritis
Skin and subcutaneous tissue disorders: eczema, rash, urticaria
Treatment of H. pylori Infection
The safety of VOQUEZNA, amoxicillin and clarithromycin was evaluated in 675 adult patients (aged 20 to 82 years) in clinical trials in the United States, Europe, and Japan, and VOQUEZNA and amoxicillin was evaluated in 348 adult patients (aged 20 to 80 years) in a clinical trial in the United States and Europe. All of the patients were screened and found to be positive for H. pylori infection.
The safety of VOQUEZNA, amoxicillin, and clarithromycin (triple therapy) and VOQUEZNA and amoxicillin (dual therapy) was evaluated in a randomized, controlled, double-blind (triple therapy)/open-label (dual therapy) study conducted in the United States and Europe in treatment-naïve H. pylori-positive adult patients [see Clinical Studies (14.4)].
Adverse Reactions Leading to Discontinuation
Treatment discontinuation due to an adverse reaction occurred in 2.3% (8/346) of the patients treated with VOQUEZNA, amoxicillin, and clarithromycin; 0.9% (3/348) of the patients treated with VOQUEZNA and amoxicillin; and 1.2% (4/345) of the patients treated with lansoprazole, amoxicillin, and clarithromycin. The most common adverse reactions leading to discontinuation of VOQUEZNA, amoxicillin, and clarithromycin were diarrhea (0.6%) and hypertension (0.6%), and the most common adverse reaction leading to discontinuation of VOQUEZNA and amoxicillin was rash (0.6%).
Most Common Adverse Reactions
Adverse reactions reported in at least 2% of patients in any treatment arm are described in Table 8.
Table 8: Adverse Reactions* in Adult Patients with H. pylori Infection† Adverse Reactions VOQUEZNA and Amoxicillin VOQUEZNA, Amoxicillin, and Clarithromycin Lansoprazole, Amoxicillin, and Clarithromycin N=348
%N=346
%N=345
%Diarrhea 5 4 10 Dysgeusia‡ 1 5 6 Vulvovaginal candidiasis‡ 2 3 1 Abdominal pain‡ 3 2 3 Headache 1 3 1 Hypertension‡ 1 2 1 Nasopharyngitis 2 <1 1 Less Common Adverse Reactions
Other adverse reactions reported in less than 2% of patients treated with VOQUEZNA, amoxicillin, and clarithromycin or VOQUEZNA and amoxicillin are listed below by body system:
Blood and lymphatic system disorders: anemia, leukocytosis, leukopenia, neutropenia
Cardiac disorders: QT prolongation, tachycardia
Eye disorders: orbital edema
Gastrointestinal disorders: abdominal distension, constipation, dry mouth, duodenal polyp, duodenal ulcer, dyspepsia, flatulence, gastric ulcer, gastroesophageal reflux disease, hematochezia, large intestine polyp, rectal polyp, nausea, stomatitis, tongue discomfort, vomiting
General disorders and administration site conditions: fatigue, pyrexia
Immune system disorders: drug hypersensitivity
Infections and infestations: anal fungal infection, gastrointestinal viral infection, oral fungal infection, pneumonia, tongue fungal infection, upper respiratory tract infection, urinary tract infection, viral infection
Investigations: increased liver function test
Metabolism and nutrition disorders: decreased appetite
Musculoskeletal system: bone fracture
Nervous system disorders: ageusia, dizziness, tension headache
Psychiatric disorders: anxiety, depression, insomnia
Renal and urinary disorders: renal hypertrophy, tubulointerstitial nephritis
Reproductive system and breast disorders: vaginal discharge
Respiratory, thoracic and mediastinal disorders: cough, nasal polyps, oropharyngeal pain
Skin and subcutaneous tissue disorders: dermatitis, dry skin, rash
For more information on adverse reactions and laboratory changes with amoxicillin or clarithromycin, refer to the Adverse Reactions section of the corresponding prescribing information.
6.2 Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of vonoprazan outside of the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: thrombocytopenia
Immune system disorders: anaphylactic shock [see Contraindications (4)]
Infections and infestations: C. difficile (with concomitant antibacterials)
Investigation: hypomagnesemia, hypokalemia, hypocalcemia, vitamin B12 deficiency
Hepatobiliary disorders: hepatic injury, hepatic failure, jaundice
Skin and subcutaneous tissue disorders: drug eruption, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis
-
7 DRUG INTERACTIONS
Table 9 and Table 10 include drugs with clinically important drug interactions and interaction with diagnostics when administered concomitantly with VOQUEZNA and instructions for preventing or managing them.
These recommendations are based on either drug interaction trials or predicted interactions due to the expected magnitude of interaction and potential for serious adverse reactions or loss of efficacy [see Clinical Pharmacology (12.3)].
Consult the labeling of concomitantly used drugs to obtain further information about interactions with vonoprazan.
Table 9: Drug Interactions Affecting Drugs Co-Administered with VOQUEZNA and Interactions with Diagnostics Drugs Dependent on Gastric pH for Absorption Antiretrovirals Clinical Effect
Vonoprazan reduces intragastric acidity [see Clinical Pharmacology (12.2)], which may alter the absorption of antiretroviral drugs, leading to changes in the safety and/or effectiveness. Prevention or Management Rilpivirine-containing products Concomitant use with VOQUEZNA is contraindicated. Atazanavir Avoid concomitant use with VOQUEZNA. Nelfinavir Other antiretrovirals See the prescribing information of other antiretroviral drugs dependent on gastric pH for absorption prior to concomitant use with VOQUEZNA. Other Drugs (e.g., iron salts, erlotinib, dasatinib, nilotinib, mycophenolate mofetil, ketoconazole/itraconazole) Clinical Effect Vonoprazan reduces intragastric acidity [see Clinical Pharmacology (12.2)], which may decrease the absorption of drugs reducing their effectiveness. Prevention or Management See the prescribing information for other drugs dependent on gastric pH for absorption. Combination Therapy with Clarithromycin and/or Amoxicillin Clinical Effect Concomitant administration of clarithromycin with other drugs can lead to serious adverse reactions, including potentially fatal arrhythmias, and is contraindicated. Amoxicillin also has drug interactions. Prevention or Management See Contraindications and Warnings and Precautions in the prescribing information for clarithromycin.
See Drug Interactions in the prescribing information for amoxicillin.Certain CYP3A Substrates Where Minimal Concentration Changes May Lead to Serious Toxicities Clinical Effect Vonoprazan is a weak CYP3A inhibitor [see Clinical Pharmacology (12.3)].
Vonoprazan may increase exposure of CYP3A4 substrates, which may increase the risk of adverse reactions related to these substrates.Prevention or Management Frequently monitor concentrations and/or adverse reactions related to the substrate drugs when used with VOQUEZNA. Dosage reduction of substrate drugs may be needed.
See prescribing information for the relevant substrate drugs.CYP2C19 Substrates (e.g., clopidogrel, citalopram, cilostazol) Clinical Effect Vonoprazan is a CYP2C19 inhibitor [see Clinical Pharmacology (12.3)]. Vonoprazan may reduce plasma concentrations of the active metabolite of clopidogrel and may cause reduction in platelet inhibition. Vonoprazan may increase exposure of CYP2C19 substrate drugs (e.g., citalopram, cilostazol). Prevention or Management Clopidogrel Carefully monitor the efficacy of clopidogrel and consider alternative anti-platelet therapy. Citalopram and Cilostazol Carefully monitor patients for adverse reactions associated with citalopram and cilostazol. See the prescribing information for dosage adjustments. Chromogranin Test for Neuroendocrine Tumors Clinical Effect Vonoprazan reduces intragastric acidity [see Clinical Pharmacology (12.2)], which increases CgA levels and may cause false positive results in diagnostic investigations for neuroendocrine tumors. Prevention or Management Assess CgA levels at least 4 weeks after stopping VOQUEZNA treatment and repeat the test if initial CgA levels are high. If serial tests are performed (e.g., for monitoring), use the same commercial laboratory for testing, as reference ranges between tests may vary. Interaction with Secretin Stimulation Test Clinical Effect Hyper-response in gastrin secretion in response to secretin stimulation test, falsely suggesting gastrinoma. Prevention or Management Temporarily stop VOQUEZNA at least 4 weeks before assessing to allow gastrin levels to return to normal [see Clinical Pharmacology (12.2)]. Table 10: Drug Interactions Affecting VOQUEZNA When Co-Administered with Other Drugs Strong or Moderate CYP3A4 Inducers Clinical Effect Vonoprazan is a CYP3A substrate. Strong or moderate CYP3A inducers decrease vonoprazan exposure [see Clinical Pharmacology (12.3)], which may reduce the effectiveness of VOQUEZNA. Prevention or Management Avoid concomitant use with VOQUEZNA. -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to VOQUEZNA during pregnancy. Healthcare providers are encouraged to register patients by calling 1-866-609-1612 or visiting https://voqueznapregnancyregistry.com/.
Risk Summary
There are no adequate and well-controlled studies of vonoprazan in pregnant women. Available data from pharmacovigilance reports with vonoprazan-containing products used in pregnant women are not sufficient to evaluate for a drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes.
In pregnant rats, no adverse effects were noted after oral administration of vonoprazan during organogenesis at approximately 27-times the maximum recommended human dose (MRHD), based on AUC exposure comparisons.
In a pre- and postnatal development (PPND) study, pups from dams orally administered vonoprazan during organogenesis and through lactation exhibited liver discoloration, which, in follow-up mechanistic animal studies, was associated with necrosis, fibrosis, and hemorrhage at a dose approximately 22-times the MRHD, based on AUC comparisons that were likely attributable to exposure during lactation [see Use in Specific Populations (8.2)]. These effects were not observed at the next lower dose in this study, which was approximately equal to the MRHD, based on AUC comparison; however, they were seen at clinically relevant exposures in dose range-finding studies in rats (see Data).
The background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defects, loss, or other adverse outcomes. In the United States general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
Pregnant rats were orally administered vonoprazan at doses of 30, 100, or 300 mg/kg/day (7-, 27-, 130-times the MRHD based on AUC comparison at the same doses from unmated female rats from separate studies) during the period of organogenesis from gestation day (GD) 6 to 17. During maternal dosing, one high-dose female died and decreased body weight and food consumption occurred at the middle and highest doses. No embryo-fetal lethality was observed but decreased fetal body weight was observed in the highest dose group. Fetal abnormalities were limited to the 300 mg/kg/day and included ventricular septal defect and mal-positioned subclavian artery in fetuses in a majority (15/19) of litters, as well as tail abnormalities and small anal opening. No adverse embryo-fetal effects were observed at the 100 mg/kg/day.
Pregnant rabbits were orally administered vonoprazan at doses of 3, 10, or 30 mg/kg/day (0.04-, 1.5-, 10-times the MRHD based on AUC comparison) during the period of organogenesis from GD 6 to 18. Two animals aborted at the highest dose and decreased body weight and food consumption occurred at the mid and high doses. No embryo-fetal mortality or toxicity occurred. There were no external, visceral, or skeletal abnormalities.
In a PPND study, pregnant female rats were orally administered vonoprazan at doses of 1, 3, 10, or 100 mg/kg/day (0.01-, 0.18-, 1.1-, 22-times the MRHD based on AUC comparison) from GD 6 to lactation day (LD) 21. Decreased body weight gain and food consumption were present in dams at the highest dose during lactation. Decreased body weight gain compared to controls was observed in the offspring from dams in the high dose group. Liver discoloration occurred in offspring from the high dose group at LD 4 but was not present in animals examined after weaning. Similarly, in dose range-finding studies in rats and follow-up mechanistic animal studies, the liver discoloration was observed and characterized as necrosis, fibrosis, and hemorrhage at equal to or greater than clinically relevant exposures based on AUC comparisons. The mechanistic studies further demonstrated the effect was likely attributable to vonoprazan exposure during lactation [see Use in Specific Populations (8.2)]. The clinical relevance of the liver findings is uncertain.
Exposure margins from vonoprazan between the animal and clinical studies for vonoprazan, amoxicillin, and clarithromycin used in combination may be lower due to increased vonoprazan exposure from concomitant use with clarithromycin in patients [see Clinical Pharmacology (12.3)].
8.2 Lactation
Risk Summary
Data from a clinical lactation study indicate that vonoprazan is present in human breast milk in amounts less than 0.03% of the administered maternal dose. Assuming an infant body weight of 6 kg and an exclusively breastfed infant, the estimated mean daily infant dose was less than 0.3% of the maternal weight-adjusted dose (see Data). There are no data on the effects of vonoprazan on the breastfed child or the effects on milk production. In animal studies, liver injury occurred in offspring from pregnant and lactating rats administered oral vonoprazan at AUC exposures approximately equal to and greater than the MRHD (see Data).
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for VOQUEZNA and any potential adverse effects on the breastfed child from VOQUEZNA or from the underlying maternal condition.
Data
Human Data
Concentrations of vonoprazan were measured in breast milk from 15 healthy lactating women following administration of vonoprazan 20 mg once daily (N=5) or twice daily (N=10) for 4 days (not an approved duration for VOQUEZNA).
Following administration of a 20 mg vonoprazan once daily regimen, the mean average concentration (Cavg) of vonoprazan in breast milk at steady state was 7.55 ng/mL. The estimated mean total amount of vonoprazan present in breast milk over 24 hours was 0.0024 mg. Assuming an infant body weight of 6 kg and an exclusively breastfed infant, the estimated mean daily infant dose was 0.0004 mg/kg/day, representing 0.13% of the maternal weight-adjusted dose.
Following administration of a 20 mg vonoprazan twice daily dosage regimen, the mean average concentration (Cavg) of vonoprazan in breast milk at steady state was 13.3 ng/mL. The estimated mean total amount of vonoprazan present in breast milk over 24 hours was 0.009 mg. Assuming an infant body weight of 6 kg and an exclusively breastfed infant, the estimated mean daily infant dose was 0.0015 mg/kg/day, representing 0.27% of the maternal weight-adjusted dose.
Animal Data
In a PPND study in rats, in which the dams were administered oral vonoprazan during gestation and through lactation at up to 22-times the MRHD (based on a comparison of AUC), liver discoloration occurred in offspring from the high dose group [see Use in Specific Populations (8.1)].Liver discoloration associated with necrosis, fibrosis, and hemorrhage in the offspring of dosed rats was also seen in dose-range finding studies and limited, non-standard, follow-up, mechanistic studies, including offspring in lactation only studies. These effects were reported in pups on LD 4 at doses from 3 to 100 mg/kg/day (approximately 0.2-to 22-fold the MRHD based on an AUC values extrapolated from the PPND study) and on LD 14 at doses from 10 to 100 mg/kg/day (approximately 1-to 22-fold the MRHD based on an extrapolated AUC comparisons). In mechanistic studies, liver effects were observed in offspring treated only during lactation but not in offspring from animals only treated during gestation. In some of these studies, this finding was associated with increased offspring stomach weights that was reversed along with liver discoloration by concomitant treatment with a gastrointestinal prokinetic agent.
8.4 Pediatric Use
The safety and effectiveness of VOQUEZNA have not been established in pediatric patients.
8.5 Geriatric Use
There were 200 patients aged 65 years and older in the clinical trial for healing and maintenance of healed erosive esophagitis [see Clinical Studies (14.1)]. Of the total number of vonoprazan-treated patients, there were 93 (18%) patients aged 65 years of age and older and 10 (2%) patients aged 75 years of age and older.
There were 139 patients aged 65 years and older in the clinical trial for the relief of heartburn associated with non-erosive gastroesophageal reflux disease [see Clinical Studies (14.3)]. Of the total number of vonoprazan-treated patients, there were 93 (18%) patients aged 65 years of age and older and 14 (3%) patients aged 75 years of age and older.
There were 218 patients aged 65 years and older in the clinical trial for the treatment of H. pylori infection [see Clinical Studies (14.4)]. Of the total number of vonoprazan-treated patients, there were 153 (22%) patients aged 65 years of age and older and 18 (3%) patients aged 75 years of age and older.
No overall differences in safety or effectiveness were observed between these patients and younger adult patients, and other reported clinical experience has not identified differences in responses between the geriatric and younger adult patients, but greater sensitivity of some older individuals cannot be ruled out.
No clinically meaningful differences in the pharmacokinetics of vonoprazan are predicted in patients 65 years of age and older compared to younger adult patients [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Healing of Erosive Esophagitis
No dosage adjustment of VOQUEZNA for the healing of erosive esophagitis is recommended in patients with mild to moderate renal impairment (eGFR 30 to 89 mL/min). Dosage reduction is recommended in patients with severe renal impairment (eGFR < 30 mL/min) [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
Maintenance of Healed Erosive Esophagitis or Relief of Heartburn Associated with Non-Erosive Gastroesophageal Reflux Disease
No dosage adjustment of VOQUEZNA for the maintenance of healed erosive esophagitis or the relief of heartburn associated with non-erosive gastroesophageal reflux disease is recommended in patients with any degree of renal impairment.
Treatment of H. pylori Infection
Use of VOQUEZNA is not recommended for the treatment of H. pylori infection in patients with severe renal impairment (eGFR < 30 mL/min) [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
Healing of Erosive Esophagitis
No dosage adjustment of VOQUEZNA for the healing of erosive esophagitis is recommended in patients with mild hepatic impairment (Child-Pugh A). Dosage reduction is recommended in patients with moderate to severe hepatic impairment (Child-Pugh Class B and C) [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
Maintenance of Healed Erosive Esophagitis or Relief of Heartburn Associated with Non-Erosive Gastroesophageal Reflux Disease
No dosage adjustment of VOQUEZNA for the maintenance of healed erosive esophagitis or the relief of heartburn associated with non-erosive gastroesophageal reflux disease is recommended in patients with any degree of hepatic impairment.
Treatment of H. pylori Infection
Use of VOQUEZNA is not recommended for the treatment of H. pylori infection in patients with moderate to severe hepatic impairment (Child-Pugh Class B and C) [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
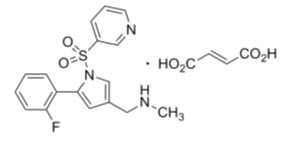
Vonoprazan (as the fumarate), is a potassium-competitive acid blocker. Chemically, it is 1H-pyrrole-3-methanamine, 5-(2-fluorophenyl)-N-methyl-1-(3-pyridinylsulfonyl)-, (2E)-2-butenedioate (1:1). Its empirical formula is C17H16FN3O2S•C4H4O4 with a molecular weight of 461.5. Vonoprazan fumarate has the following structure:
Vonoprazan fumarate is white to nearly white crystals or crystalline powder, which melts at 194.8°C. Vonoprazan fumarate is soluble in dimethyl sulfoxide; sparingly soluble in N,N–dimethylacetamide, slightly soluble in N,N-dimethylformamide, methanol, and water; very slightly soluble in ethanol (99.5%); and practically insoluble in 2-propanol, acetone, 1-octanol, and acetonitrile.
VOQUEZNA (vonoprazan) tablets are available in two dosage strengths for oral administration: 10 mg of vonoprazan (equivalent to 13.36 mg of vonoprazan fumarate) and 20 mg of vonoprazan (equivalent to 26.72 mg of vonoprazan fumarate). Each film-coated tablet contains the following inactive ingredients: ascorbic acid, croscarmellose sodium, ferric oxide red (only in 20 mg tablets), ferric oxide yellow (only in 10 mg tablets), fumaric acid, hydroxypropyl cellulose, hypromellose, magnesium stearate, mannitol, microcrystalline cellulose, polyethylene glycol 8000, and titanium dioxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Vonoprazan suppresses basal and stimulated gastric acid secretion at the secretory surface of the gastric parietal cell through inhibition of the H+, K+-ATPase enzyme system in a potassium-competitive manner. Because this enzyme is regarded as the acid (proton) pump within the parietal cell, vonoprazan has been characterized as a type of gastric proton-pump inhibitor, in that it blocks the final step of acid production. Vonoprazan does not require activation by acid. Vonoprazan may selectively concentrate in the parietal cells in both the resting and stimulated states. Vonoprazan binds to the active pumps in a noncovalent and reversible manner.
12.2 Pharmacodynamics
Antisecretory Activity
Following a single 10 mg or 20 mg dose of vonoprazan, the onset of the antisecretory effect, as measured by intragastric pH, occurs within 2 to 3 hours. The elevated intragastric pH levels compared to placebo increase with dose and are maintained for over 24 hours after dosing. The inhibitory effect of vonoprazan on acid secretion increases with repeated daily dosing and steady state is achieved by Day 4. The antisecretory effect of vonoprazan decreases following drug discontinuation although intragastric pH remained elevated compared to placebo for 24 to 48 hours following the dose on Day 7.
The effects of vonoprazan 10 mg or 20 mg once daily for 7 days on 24-hour intragastric pH in healthy subjects are shown in Table 11.
Table 11: Effect of VOQUEZNA 10 mg or 20 mg Once Daily on 24-Hour Intragastric pH at Baseline and on Days 1 and 7 in Healthy Subjects Parameter VOQUEZNA
10 mg Once Daily
(N=9)VOQUEZNA
20 mg Once Daily
(N=9)Baseline Day 1 Day 7 Baseline Day 1 Day 7 Mean Intragastric pH 2.0 3.7 4.6 1.9 4.5 5.9 % Time Intragastric pH>4 (hours) 6.8
(2 h)43.1
(10 h)60.2
(14 h)7.4
(2 h)62.7
(15 h)85.2
(20 h)% Time Intragastric pH>6 (hours) 1.3
(<1 h)20.7
(5 h)34.3
(8 h)0.9
(<1 h)29.0
(7 h)57.8
(14 h)Cardiac Electrophysiology
At a single dose of 120 mg (6-times the maximum recommended dose), vonoprazan does not prolong the QT interval to any clinically relevant extent.
Serum Gastrin Effects
The effect of vonoprazan on serum gastrin concentrations was evaluated in clinical trials for the healing of erosive esophagitis, maintenance of healed erosive esophagitis, and relief of heartburn associated with non-erosive gastroesophageal reflux disease [see Clinical Studies (14)]. In patients with erosive esophagitis treated with VOQUEZNA 20 mg once daily, the mean fasting gastrin levels at Week 2 increased from baseline and levels were similar at Week 2 and Week 8. During maintenance treatment for healed erosive esophagitis with VOQUEZNA 10 mg once daily, the mean gastrin levels remained elevated and returned to normal within 4 weeks of discontinuation of treatment.
In patients with non-erosive gastroesophageal reflux disease treated with VOQUEZNA 10 mg once daily, the mean fasting gastrin levels increased from baseline, remained elevated during treatment, and returned to normal within 4 weeks of discontinuation of treatment.
Increased gastrin causes enterochromaffin-like cell hyperplasia and increased serum CgA levels. The increased CgA levels may cause false positive results in diagnostic investigations for neuroendocrine tumors [see Warnings and Precautions (5.8) and Drug Interactions (7)].
Enterochromaffin-Like Cell (ECL) Effects
Human gastric biopsy specimens were obtained from 135 patients treated with vonoprazan 10 mg or 20 mg once daily for up to 260 weeks. An increase in the incidence of hyperplasia of the parietal cells and G-cells was observed, which is consistent with the pharmacological action of a potassium-competitive acid blocker. No neoplastic changes were observed [see Nonclinical Toxicology (13.1), (13.2)].
12.3 Pharmacokinetics
Steady state pharmacokinetic (PK) parameters for vonoprazan 10 mg or 20 mg following once daily administration and vonoprazan 20 mg following twice daily administration from data collected across multiple studies are summarized in Table 12.
Table 12: Mean (%CV) Steady State Pharmacokinetic Parameters For Vonoprazan Following Once or Twice Daily Dosing PK Parameter Vonoprazan 10 mg Vonoprazan 20 mg Once Daily
(N=30)Once Daily
(N=68)Twice Daily
(N=32)Tmax(h) median (min, max) 1.5 (0.75, 3.0) 2.0 (0.75, 5.0) 3.0 (1.0-6.0) Cmax (ng/mL) 11.7 (27.5) 26.1 (35.2) 37.8 (36.1) AUC (hr*ng/mL) 92.9 (33.1)* 230.9 (41.3)* 272.5 (30.5)† t1/2z (h) 7.7 (27.1) 7.9 (22.6) 6.8 (22.7) CL/F (L/h) 120.2 (35.2) 100.2 (38.3) 81.3 (35.7) Vz/F (L) 1270.7 (26.6) 1114.0 (39.6) 782.7 (34.4) Cmax = Maximum plasma concentration; AUC0-24h = Area under the plasma concentration-time curve from time 0 to end of the 24-hour dosing interval; AUC0-12h = Area under the plasma concentration-time curve from time 0 to the end of the 12-hour dosing interval; Tmax = Time to reach Cmax, t1/2 = Elimination half-life, CL/F = Apparent oral clearance, Vz/F = Apparent oral volume of distribution
Absorption
Vonoprazan exhibits time-independent pharmacokinetics and steady state concentrations are achieved by Day 3 to 4. After multiple doses of vonoprazan ranging from 10 to 40 mg (twice the maximum recommended dose) once daily for 7 days in healthy subjects, Cmax and area under the plasma concentration-time curve (AUC) values for vonoprazan increased in an approximately dose-proportional manner.
There is little accumulation in plasma after once daily multiple doses, with an accumulation index ratio of less than 1.2 based on AUC for doses ranging from 10 to 40 mg (twice the maximum recommended dose).
Steady state plasma exposure of vonoprazan following 20 mg twice daily dosing (AUC0-12h = 273 hr*ng/mL, N=10) was approximately 1.8-fold higher compared to the mean estimate from the same subjects on Day 1 (AUC0-12h = 155 hr*ng/mL, N=10).
Effect of Food
In a food effect study in healthy subjects (N=24) who received vonoprazan 20 mg, a high-fat meal resulted in a 5% increase in Cmax, a 15% increase in AUC, and a delay in median Tmax of 2 hours. These changes are not considered to be clinically significant [see Dosage and Administration (2.4)].
Distribution
Plasma protein binding of vonoprazan ranged from 85 to 88% in healthy subjects and was independent of concentration from 0.1 to 10 mcg/mL.
Elimination
Metabolism
Vonoprazan is metabolized to inactive metabolites via multiple pathways by a combination of cytochrome P450 (CYP) isoforms (predominantly CYP3A4/5, CYP2C19, CYP2D6, and CYP2B6) along with sulfo- and glucuronosyl-transferases. CYP2C19 and CYP2D6 polymorphisms have been evaluated in clinical studies and there were no clinically meaningful differences in the pharmacokinetics of vonoprazan based on either CYP2C19 or CYP2D6 metabolizer status.
Specific Populations
Geriatric Patients
No clinically meaningful differences in the pharmacokinetics of vonoprazan are predicted in patients 65 years of age and older compared to younger adult patients.
Sex, Race, or Ethnicity
There were no clinically significant differences in the pharmacokinetics of vonoprazan based on sex or race/ethnicity.
Patients with Renal impairment
The pharmacokinetics of vonoprazan administered as a single 20 mg dose in patients with mild [eGFR 60 to <90 mL/min/1.73 m2 (N=8)], moderate [eGFR 30 to <60 mL/min/1.73 m2 (N=8)], or severe [eGFR 15 to <30 mL/min/1.73 m2 (N=8)] renal impairment were compared to those with normal renal function [eGFR ≥90 mL/min/1.73 m2 (N=13)]. Compared to subjects with normal renal function, systemic exposure (AUC0-inf) was 1.7-, 1.3-, and 2.4-times greater in patients with mild, moderate, and severe renal impairment, respectively. In subjects requiring dialysis (N=8), AUC0-inf estimates were 1.3-fold greater compared to estimates from subjects with normal renal function [see Dosage and Administration (2.2)]. Protein binding of vonoprazan is not affected by impaired renal function. In patients requiring dialysis, vonoprazan was present in the dialysate and represented 0.94% of the dose administered.
Patients with Hepatic Impairment
The pharmacokinetics of vonoprazan administered as a single 20 mg dose in patients with mild [Child-Pugh Class A (N=8)], moderate [Child-Pugh Class B (N=8)], or severe [Child-Pugh Class C (N=6)] hepatic impairment were compared to those with normal hepatic function (N=12). Compared to subjects with normal hepatic function, systemic exposure (AUC0-inf) of vonoprazan was 1.2-, 2.4-, and 2.6-times greater in patients with mild, moderate, and severe hepatic impairment, respectively [see Dosage and Administration (2.3)]. Protein binding of vonoprazan is not affected by impaired hepatic function.
Drug Interaction Studies
Clinical Studies
Combination Therapy with Vonoprazan, Amoxicillin, and Clarithromycin
When vonoprazan 20 mg, amoxicillin 750 mg, and clarithromycin 400 mg were co-administered twice daily for 7 days (N=11), there was no effect on pharmacokinetics of amoxicillin compared to amoxicillin alone. However, vonoprazan Cmax and AUC0-12h increased by 87% and 85%, respectively, and clarithromycin Cmax and AUC0-12h increased by 64% and 45%, respectively, compared to administration of each component alone.
Effect of Vonoprazan on CYP3A4 Substrates
When a single oral dose of midazolam 2 mg was administered following vonoprazan 20 mg twice daily for 7 days (N=20), midazolam AUC0-inf increased 93% compared to administration of midazolam alone.
Effect of CYP3A Inhibitors on Vonoprazan
When a single dose of 40 mg vonoprazan (twice the maximum recommended dose) was administered with clarithromycin 500 mg twice daily for 7 days (N=16), vonoprazan AUC0-inf increased 58% compared to administration of vonoprazan alone.
Coadministration of Vonoprazan with Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) or Low-Dose Aspirin
When a single dose of 40 mg vonoprazan (twice the maximum recommended dose) was co-administered with diclofenac 25 mg, meloxicam 10 mg, or aspirin 100 mg, there were no clinically meaningful changes in exposure of vonoprazan, diclofenac, meloxicam, or aspirin compared to administration of each drug alone.
12.4 Microbiology
Antimicrobial Activity
Culture and sensitivity testing of bacteria are not routinely performed to establish the diagnosis of H. pylori infection [see Clinical Studies (14.4)]. The following in vitro data are available, but their clinical significance is unknown. Clarithromycin and amoxicillin are active in vitro against most isolates of H. pylori.
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
In a 24-month carcinogenicity study in mice, vonoprazan at daily oral doses of 6, 20, 60, and 200 mg/kg/day (approximately 0.4-, 4-, 19-, and 93-times the MRHD based on AUC) produced hyperplasia of neuroendocrine cells, gastropathy, and benign and/or malignant neuroendocrine cell tumors (carcinoids) in the stomach at all doses in males and at 60 mg/kg/day and greater in females. In the liver, increased incidences of hepatocellular adenoma and carcinomas were observed at doses of 20 mg/kg/day and greater in males and 60 mg/kg/day and greater in females.
In a 24-month carcinogenicity study in Sprague-Dawley rats, vonoprazan at daily oral doses of 5, 15, 50, and 150 mg/kg/day (approximately 0.6-, 4-, 19-, and 65-times the MRHD based on AUC) produced benign and/or malignant neuroendocrine cell tumors in the stomach in both male and female rats at doses of 5 mg/kg/day or more. Increased incidence of hepatocellular adenoma and carcinomas and hepatocholangiocellular adenomas and carcinomas were observed at doses of 50 and 150 mg/kg/day.
In both mice and rats, neuroendocrine tumors in the stomach occurred in association with neuroendocrine hyperplasia, and gastropathy in the stomach and increased plasma gastrin concentrations that are consistent with inhibition of gastric acid secretion. Carcinoid tumors have also been observed in rats subjected to fundectomy or long-term treatment with PPIs or high doses of H2-receptor antagonists.
Mutagenesis
Vonoprazan was negative for mutagenicity in the in vitro Ames test, in an in vitro clastogenicity assay in Chinese Hamster cells and in vivo in a rat bone marrow micronucleus study.
Impairment of Fertility
Vonoprazan at oral doses up to 300 mg/kg/day in rats (approximately 133-times the MRHD based on AUC from a separate study in nonpregnant animals administered the same dose) was found to have no effect on fertility and reproductive performance. Elongation of the estrous cycle was observed in rats at doses equivalent to 133-times the MRHD, based on AUC.
13.2 Animal Toxicology and/or Pharmacology
During lifetime exposure of mice and rats dosed daily with up to 200 mg/kg/day and 150 mg/kg/day of vonoprazan, respectively, increases in gastrin levels and marked neuroendocrine hyperplasia and gastropathy were observed, followed by formation of carcinoid tumors [see Nonclinical Toxicology (13.1)]. This finding is considered to be a rodent-specific phenomenon.
-
14 CLINICAL STUDIES
14.1 Healing of Erosive Esophagitis and Relief of Heartburn
The effectiveness and safety of VOQUEZNA was evaluated in a randomized, active-controlled, double-blind, eight-week study conducted in the United States and Europe in 1024 adult patients with endoscopically confirmed erosive esophagitis (NCT04124926). Severity of the disease was classified based on the Los Angeles (LA) Classification Grading System (Grades A through D). Patients were randomized to one of the following treatment groups: VOQUEZNA 20 mg once daily or lansoprazole 30 mg once daily for 2 to 8 weeks. Patients who were positive for H. pylori infection or who had Barrett's esophagus and/or definite dysplastic changes in the esophagus at baseline were excluded from the study. Based on the LA Classification, 66% of patients had mild erosive esophagitis (Grades A or B) and 34% of patients had moderate to severe erosive esophagitis (Grades C or D) prior to randomization. Patients in the trial had a mean age of 51 years (range 18 to 84 years), 53% were female, 12% identified as Hispanic or Latino, 91% identified as White, 6% as Black or African American, and 3% identified as another racial group.
Healing of erosive esophagitis was assessed at Week 2 and Week 8 and resolution of heartburn symptoms was evaluated daily over the 8-week period. If endoscopic healing of erosive esophagitis was confirmed at Week 2, the patient entered the maintenance phase of the study. If endoscopic healing was not confirmed at Week 2, the patient continued to receive randomized treatment until Week 8. Only patients with confirmed endoscopic healing entered the maintenance phase. All endoscopies were centrally read and adjudicated.
Healing of All Grades of Erosive Esophagitis
The primary endpoint was endoscopically confirmed complete healing of all grades of erosive esophagitis at Week 2 or Week 8, as shown in Table 13.
Table 13: Rates of Healing of All LA Grades of Erosive Esophagitis in Adults at Week 2 or Week 8 Timepoint Treatment Group Treatment Difference
(95% Confidence Interval)VOQUEZNA
20 mg Once DailyLansoprazole
30 mg Once DailyN=514
%N=510
%- *
- Demonstrated non-inferiority to lansoprazole.
Week 2 or 8 93 85 8*
(4.5, 12.2)Week 2 74 68 Healing of Erosive Esophagitis in Subgroups with LA Grade C or D Esophagitis
For the secondary endpoint of complete healing of erosive esophagitis at Week 2, superiority was demonstrated in the subgroup of patients with LA Grade C or D disease, 70% of 177 VOQUEZNA-treated patients and 53% of 174 lansoprazole-treated patients achieved healing (18% treatment difference; 95% CI 7.4, 27.4).
Complete healing of erosive esophagitis at either Week 2 or Week 8 in the subgroup of patients with LA Grade C or D disease was 92% in patients treated with VOQUEZNA and 72% in patients treated with lansoprazole. This endpoint was not statistically significant under the prespecified multiple testing procedure.
Relief of Heartburn in Patients with Erosive Esophagitis During the Healing Phase
The percentage of 24-hour heartburn-free days through Week 8 was evaluated as a secondary endpoint and results are shown in Table 14.
Table 14: Percentage of 24-Hour Heartburn-Free Days in Adults with Erosive Esophagitis Through Week 8 Parameter Treatment Group Treatment Difference
(95% Confidence Interval)VOQUEZNA
20 mg Once Daily
N=514
%Lansoprazole
30 mg Once Daily
N=510
%- *
- Demonstrated non-inferiority to lansoprazole.
Mean ± SD 67 ± 35 64 ± 35 3*
(-1.6, 7.0)Median 81 78 Other Healing of Erosive Esophagitis Studies
Two additional randomized, active-controlled, double-blind studies conducted outside of the United States, of similar design to the United States trial, also demonstrated non-inferiority of vonoprazan 20 mg once daily compared to lansoprazole 30 mg once daily for the primary endpoint of healing of all grades of erosive esophagitis by Week 8.
14.2 Maintenance of Healed Erosive Esophagitis and Relief of Heartburn
Patients who completed the healing phase of the erosive esophagitis study (NCT04124926) and showed endoscopically confirmed healed erosive esophagitis at Week 2 or Week 8 were re-randomized in the maintenance phase 1:1:1 to either VOQUEZNA 10 mg once daily, a higher dosage of VOQUEZNA, or lansoprazole 15 mg once daily. Maintenance of healing and resolution of heartburn symptoms were evaluated over 24 weeks. The higher VOQUEZNA dose group did not demonstrate additional treatment benefit compared to VOQUEZNA 10 mg once daily.
Maintenance of Healed Erosive Esophagitis
The primary endpoint was maintenance of healed erosive esophagitis (all grades) through Week 24. A secondary endpoint was maintenance of healed erosive esophagitis in the subgroup of patients with LA Grade C or D disease prior to randomization in the healing phase of the study.
The maintenance rates of healed erosive esophagitis are shown in Table 15.
Table 15: Maintenance Rates of Healed Erosive Esophagitis in Adults Through Week 24 Baseline Severity Treatment Group Treatment Difference
(95% Confidence Interval)VOQUEZNA
10 mg Once DailyLansoprazole
15 mg Once DailyAll LA Grades: N=293 N=294 Week 24 79% 72% 7*
(0.2, 14.1)LA Grade C or D: N=95 N=96 Week 24 75% 61% 13†
(0.02, 26.1)Relief of Heartburn During Maintenance of Healed Erosive Esophagitis
The percentage of 24-hour heartburn-free days through Week 24 was evaluated for non-inferiority as a secondary endpoint, as shown in Table 16.
Table 16: Percentage of 24-Hour Heartburn-Free Days in Adults with Healed Erosive Esophagitis Through Week 24 Parameter Treatment Group Treatment Difference
(95% Confidence Interval)VOQUEZNA
10 mg Once Daily
N=293
%Lansoprazole
15 mg Once Daily
N=294
%- *
- Demonstrated non-inferiority to lansoprazole.
Mean ± SD 81 ± 29 79 ± 27 2*
(-2.3, 6.8)Median 95 89 Other Maintenance of Healed Erosive Esophagitis Studies
Two additional randomized, active-controlled, double-blind studies conducted outside of the United States, of similar design to the United States trial, also demonstrated non-inferiority of vonoprazan 10 mg once daily compared to lansoprazole 15 mg once daily for the primary endpoint of maintenance of healed erosive esophagitis (all grades) through Week 24.
14.3 Relief of Heartburn Associated with Non-Erosive Gastroesophageal Reflux Disease
The effectiveness and safety of VOQUEZNA was evaluated in a randomized, placebo-controlled, double-blind, four-week efficacy trial with a 20-week safety extension conducted in the United States in 772 adult patients with a diagnosis of symptomatic non-erosive gastroesophageal reflux disease (NCT05195528). Patients who identified heartburn as their primary symptom, had a history of heartburn for six months or longer, had heartburn on at least four of seven days immediately prior to randomization, were negative for H. pylori infection, and had no esophageal erosions as confirmed by endoscopy were enrolled. Patients were randomized 1:1:1 to one of the following treatment groups in the 4-week placebo-controlled phase: VOQUEZNA 10 mg once daily, a higher dosage of VOQUEZNA, or placebo once daily. The higher VOQUEZNA dose group did not demonstrate additional treatment benefit compared to VOQUEZNA 10 mg once daily through Week 4. Patients in the trial had a mean age of 51 years (range 18 to 83 years), 68% were female, 32% identified as Hispanic or Latino, 75% identified as White, 16% as Black or African American, 6% as Asian, and 3% identified as another racial group.
The primary endpoint was the percentage of 24-hour heartburn-free days, as assessed by daily diary over 4 weeks, as shown in Table 17.
Table 17: Percentage of 24-Hour Heartburn-Free Days in Patients with Non-Erosive Gastroesophageal Reflux Disease Through Week 4 Treatment Group Parameter VOQUEZNA
10 mg Once Daily
N=257
%Placebo
Once Daily
N=258
%Treatment Difference
(95% Confidence Interval)Mean = least squares mean
SE = standard errorLS Mean* (SE) 45 (2) 28 (2) 17†
(12, 22)Median 48 17 The difference in the percentage of patients who were heartburn-free during the 24-hour period on Day 2 of treatment was similar to the difference in 24-hour heartburn-free days through Week 4 in patients treated with VOQUEZNA 10 mg once daily compared to patients treated with placebo.
14.4 Treatment of Helicobacter pylori Infection
The effectiveness and safety of VOQUEZNA, amoxicillin, and clarithromycin (triple therapy) and VOQUEZNA and amoxicillin (dual therapy) were evaluated in a randomized, controlled, double-blind (triple therapy)/open-label (dual therapy) study conducted in the United States and Europe in treatment-naïve H. pylori-positive adult patients with at least one clinical condition: dyspepsia lasting at least 2 weeks, functional dyspepsia, recent/new diagnosis of peptic ulcer, peptic ulcer not treated for H. pylori infection, or a stable dose of long-term NSAID treatment (NCT04167670). Patients were randomized 1:1:1 to one of the following regimens administered for 14 consecutive days:
- VOQUEZNA 20 mg twice daily, amoxicillin 1,000 mg twice daily, and clarithromycin 500 mg twice daily
- VOQUEZNA 20 mg twice daily and amoxicillin 1,000 mg three times daily
- lansoprazole 30 mg twice daily, amoxicillin 1,000 mg twice daily, and clarithromycin 500 mg twice daily
H. pylori infection at baseline was defined as positive by 13C urea breath test (UBT) and follow-up upper endoscopy (culture or histology). H. pylori eradication was confirmed with a negative 13C UBT test-of-cure at least 27 days post-therapy. Patients with negative test results were considered treatment successes. Patients who tested positive for H. pylori infection and patients with missing results from the test-of-cure visit were considered treatment failures.
A total of 346 patients received VOQUEZNA, amoxicillin, and clarithromycin, 348 patients received VOQUEZNA and amoxicillin, and 345 patients received lansoprazole, amoxicillin, and clarithromycin. These patients had a mean age of 51 years (range 20 to 87 years), 62% were female, 27% identified as Hispanic or Latino, 89% identified as White, 7% as Black or African American, 2% as Asian, and 2% identified as another racial group.
VOQUEZNA, amoxicillin, and clarithromycin and VOQUEZNA and amoxicillin were shown to be non-inferior to lansoprazole, amoxicillin, and clarithromycin in patients who did not have a clarithromycin- or amoxicillin-resistant strain of H. pylori at baseline. VOQUEZNA, amoxicillin, and clarithromycin and VOQUEZNA and amoxicillin were shown to be superior to lansoprazole, amoxicillin, and clarithromycin in patients who had a clarithromycin-resistant strain of H. pylori at baseline and in the overall population.
H. pylori eradication rates at least 27 days post-therapy are shown in Table 18.
Table 18: Eradication Rates of H. pylori in Adult Patients at Least 27 Days Post-Therapy - mITT VOQUEZNA, Amoxicillin, and Clarithromycin VOQUEZNA and Amoxicillin Lansoprazole, Amoxicillin, and Clarithromycin (LAC) %
(n)%
(n)%
(n)CI = confidence interval calculated via the Miettinen and Nurminen method.
Modified intent to treat (mITT) population: Patients were included in the mITT analysis if they had documented H. pylori infection at baseline.- *
- Clarithromycin-resistant strains of H. pylori were considered those with an MIC ≥ 1 mcg/mL; amoxicillin-resistant strains were considered those with an MIC > 0.125 mcg/mL.
- †
- p<0.0001 for test of non-inferiority versus LAC.
- ‡
- p<0.01 for test of non-inferiority versus LAC.
- §
- p=0.0003 for test of superiority versus LAC.
- ¶
- p=0.01 for test of superiority versus LAC.
- #
- p<0.0001 for test of superiority versus LAC.
Patients with H. pylori infection who did not have a clarithromycin- or amoxicillin-resistant strain at baseline* 85
(222)79
(208)79
(201)Treatment difference from LAC (95% CI) 6†
(-0.8, 12.6)-0.3‡
(-7.4, 6.8)All randomized patients with H. pylori infection at baseline 81
(273)77
(250)69
(226)Treatment difference from LAC (95% CI) 12§
(5.7, 18.8)9¶
(1.9, 15.4)Patients with H. pylori infection who had a clarithromycin-resistant strain of H. pylori at baseline 66
(48)70
(39)32
(23)Treatment difference from LAC (95% CI) 34#
(17.7, 48.1)38#
(20.5, 52.6) -
16 HOW SUPPLIED/STORAGE AND HANDLING
VOQUEZNA (vonoprazan) tablets:
10 mg of vonoprazan: pale yellow, oval, film-coated tablets debossed V10 on one side and plain on the other side. Bottles of 30 (NDC 81520-100-30).
20 mg of vonoprazan: pale red, oval, film-coated tablets debossed V20 on one side and plain on the other side. Bottles of 30 (NDC 81520-200-30).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Advise patients of the following:
Acute Tubulointerstitial Nephritis
To call their healthcare provider if they experience signs and/or symptoms associated with acute tubulointerstitial nephritis [see Warnings and Precautions (5.2)].
Clostridioides difficile-Associated Diarrhea
To immediately call their healthcare provider if they experience diarrhea that does not improve [see Warnings and Precautions (5.3)].
Bone Fracture
To report any fractures, especially of the hip, wrist, or spine, to their healthcare provider [see Warnings and Precautions (5.4)].
Severe Cutaneous Adverse Reactions
To discontinue VOQUEZNA and report to their healthcare provider at first appearance of a severe cutaneous adverse reaction or other sign of hypersensitivity [see Warnings and Precautions (5.5)].
Vitamin B12 (Cobalamin) Deficiency
To report any clinical symptoms that may be associated with Vitamin B12 deficiency to their healthcare provider, if they have been receiving VOQUEZNA long-term [see Warnings and Precautions (5.6)].
Hypomagnesemia and Mineral Metabolism
To report any clinical symptoms that may be associated with hypomagnesemia, hypocalcemia, and/or hypokalemia to their healthcare provider [see Warnings and Precautions (5.7)].
Drug Interactions
To report to their healthcare provider if they start treatment with rilpivirine-containing products [see Contraindications (4)].
Pregnancy
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to VOQUEZNA during pregnancy [see Use in Specific Populations (8.1)].
Important Administration Instructions
- VOQUEZNA can be taken with or without food [see Dosage and Administration (2) and Clinical Pharmacology (12.3)].
- Swallow VOQUEZNA tablets whole; do not chew or crush the tablet.
- Missed doses:
- For the healing or maintenance of healed erosive esophagitis or for the relief of heartburn associated with non-erosive gastroesophageal reflux disease: If a dose is missed, administer VOQUEZNA as soon as possible within 12 hours after the missed dose. If more than 12 hours have passed, skip the missed dose and take your next dose at your regularly scheduled time [see Dosage and Administration (2)].
- For the treatment of H. pylori infection: If a dose is missed, administer VOQUEZNA as soon as possible within 4 hours after the missed dose. If more than 4 hours have passed, skip the missed dose and administer your next dose at the regularly scheduled time. Continue the normal dosing schedule until the treatment is completed [see Dosage and Administration (2)].
- SPL UNCLASSIFIED SECTION
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 11/2025 PATIENT INFORMATION
VOQUEZNA® (voe kwez nah)
(vonoprazan)
tablets, for oral useWhat is VOQUEZNA?
VOQUEZNA is a prescription medicine called a potassium-competitive acid blocker. VOQUEZNA reduces the amount of acid in your stomach.
VOQUEZNA is used in adults:- for 8 weeks to heal acid-related damage to the lining of the esophagus (called erosive esophagitis) and for relief of heartburn related to erosive esophagitis.
- for up to 6 months to maintain healing of erosive esophagitis and for relief of heartburn related to erosive esophagitis.
- for 4 weeks for relief of heartburn related to gastroesophageal reflux disease.
- for 14 days with the antibiotics amoxicillin and clarithromycin to treat an infection caused by bacteria called Helicobacter pylori (H. pylori).
- for 14 days with the antibiotic amoxicillin to treat an infection caused by bacteria called H. pylori.
It is not known if VOQUEZNA is safe and effective in children. Do not take VOQUEZNA if you are: - allergic to vonoprazan or any of the ingredients in VOQUEZNA. See the end of this Patient Information leaflet for a complete list of ingredients in VOQUEZNA. Allergic reaction symptoms may include trouble breathing, rash, itching, and swelling of your face, lips, tongue, or throat.
- taking a medicine that contains rilpivirine (EDURANT, JULUCA, ODEFSEY, COMPLERA) used to treat HIV-1 (Human Immunodeficiency Virus).
Before taking VOQUEZNA, tell your healthcare provider about all of your medical conditions, including if you: - have low magnesium, calcium, or potassium in your blood or you are taking a medicine to increase urine (diuretic).
- have kidney problems.
- have liver problems.
- are pregnant, think you may be pregnant, or plan to become pregnant. It is not known if VOQUEZNA will harm your unborn baby.
- Pregnancy Exposure Registry: There is a pregnancy registry for women who take VOQUEZNA during pregnancy. The purpose of this registry is to collect information about the health of you and your baby. If you are pregnant or become pregnant during treatment with VOQUEZNA, talk to your healthcare provider about how you can join this pregnancy registry or you can enroll in the registry by calling 1-866-609-1612 or visiting https://voqueznapregnancyregistry.com/.
- are breastfeeding or plan to breastfeed. You and your healthcare provider should decide if you will take VOQUEZNA while breastfeeding.
How should I take VOQUEZNA? - Take VOQUEZNA exactly as your healthcare provider tells you to take it.
- Do not change your dose or stop taking VOQUEZNA without talking to your healthcare provider first.
- Take VOQUEZNA with or without food.
- Swallow VOQUEZNA tablets whole. Do not chew or crush the tablet.
- For the treatment of erosive esophagitis or the relief of heartburn related to gastroesophageal reflux disease:
- If you miss a dose of VOQUEZNA, take it as soon as possible within 12 hours after the missed dose. If more than 12 hours have passed, skip the missed dose and take the next dose at the regularly scheduled time.
- For the treatment of H. pylori infection:
- If you miss a dose of VOQUEZNA, take it as soon as possible within 4 hours after the missed dose. If more than 4 hours have passed, skip the missed dose and take the next dose at the regularly scheduled time. Continue your regular dosing schedule until the treatment is completed.
What are the possible side effects of VOQUEZNA?
VOQUEZNA may cause serious side effects, including:- A type of kidney problem (acute tubulointerstitial nephritis). Some people who take VOQUEZNA may develop a kidney problem called acute tubulointerstitial nephritis. Call your healthcare provider right away if you have a decrease in the amount that you urinate or if you have blood in your urine.
- Diarrhea caused by an infection (Clostridioides difficile) in your intestines. Call your healthcare provider right away if you have watery stools, stomach pain, or fever that does not go away.
- Bone fractures (hip, wrist, or spine). Bone fractures in the hip, wrist, or spine may happen in people who take multiple daily doses of another type of medicine that reduces acid in your stomach known as proton pump inhibitors (PPI medicines) for a long period of time (a year or longer). Tell your healthcare provider if you have a bone fracture, especially in the hip, wrist, or spine.
-
Severe skin reactions.VOQUEZNA can cause rare but severe skin reactions that may affect any part of your body. These serious skin reactions may need to be treated in a hospital and may be life threatening:
- Skin rash, which may have blistering, peeling, or bleeding on any part of your skin (including your lips, eyes, mouth, nose, genital, hands, or feet).
- You may also have fever, chills, body aches, shortness of breath, or enlarged lymph nodes.
- Low Vitamin B-12 levels. VOQUEZNA lowers the amount of acid in your stomach. Stomach acid is needed to absorb Vitamin B12 properly. Tell your healthcare provider if you have symptoms of low vitamin B12 levels, including irregular heartbeat, shortness of breath, lightheadedness, tingling or numbness in the arms and legs, muscle weakness, pale skin, feeling tired, or mood changes. Talk with your healthcare provider about the risk of low Vitamin B12 levels if you have been on VOQUEZNA for a long time.
- Low magnesium levels in the body can happen in people who take VOQUEZNA. Tell your healthcare provider right away if you have symptoms of low magnesium levels, including seizures, dizziness, irregular heartbeat, jitteriness, muscle aches or weakness, or spasms of the hands, feet, or voice.
- Stomach growths (fundic gland polyps). A certain type of stomach growth called fundic gland polyps may happen in people who take VOQUEZNA for a long time (more than a year). Talk with your healthcare provider about the risk of fundic gland polyps if you have been on VOQUEZNA for a long time.
The most common side effects of VOQUEZNA for treatment of erosive esophagitis or relief of heartburn related to gastroesophageal reflux disease include: - stomach inflammation
- diarrhea
- stomach bloating
- stomach pain
- nausea
- indigestion
- constipation
- high blood pressure
- urinary tract infection
The most common side effects of VOQUEZNA when used with antibiotics for treatment of H. pylori infection include: - diarrhea
- temporary changes in sense of taste
- vaginal yeast infection
- stomach pain
- headache
- high blood pressure
- cold-like symptoms
These are not all the possible side effects of VOQUEZNA.
For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store VOQUEZNA? - Store VOQUEZNA at room temperature between 68°F to 77°F (20°C to 25°C).
General information about the safe and effective use of VOQUEZNA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use VOQUEZNA for a condition for which it was not prescribed. Do not give VOQUEZNA to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about VOQUEZNA that is written for health professionals.What are the ingredients of VOQUEZNA?
Active ingredient: vonoprazan
Inactive ingredients: ascorbic acid, croscarmellose sodium, ferric oxide red (only in 20 mg tablets), ferric oxide yellow (only in 10 mg tablets), fumaric acid, hydroxypropyl cellulose, hypromellose, magnesium stearate, mannitol, microcrystalline cellulose, polyethylene glycol 8000, and titanium dioxide.VOQUEZNA is manufactured for and distributed by:
Phathom Pharmaceuticals, Inc.
Buffalo Grove, IL 60089, U.S.A.
VOQUEZNA, the VOQUEZNA logo, and Phathom Pharmaceuticals are registered trademarks of Phathom Pharmaceuticals, Inc.
© 2025 Phathom Pharmaceuticals, Inc. All rights reserved. - PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle Label
- PRINCIPAL DISPLAY PANEL - 20 mg Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
VOQUEZNA
vonoprazan fumarate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:81520-100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength vonoprazan fumarate (UNII: 4QW3X4AMLB) (vonoprazan - UNII:1R5L3J156G) vonoprazan fumarate 13.36 mg Inactive Ingredients Ingredient Name Strength Mannitol (UNII: 3OWL53L36A) Microcrystalline Cellulose (UNII: OP1R32D61U) Hydroxypropyl Cellulose, Unspecified (UNII: 9XZ8H6N6OH) Fumaric Acid (UNII: 88XHZ13131) Ascorbic acid (UNII: PQ6CK8PD0R) Croscarmellose sodium (UNII: M28OL1HH48) Magnesium Stearate (UNII: 70097M6I30) Hypromellose, unspecified (UNII: 3NXW29V3WO) Polyethylene Glycol 8000 (UNII: Q662QK8M3B) Titanium Dioxide (UNII: 15FIX9V2JP) Ferric Oxide Yellow (UNII: EX438O2MRT) Product Characteristics Color YELLOW (pale yellow) Score no score Shape OVAL Size 8mm Flavor Imprint Code V10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81520-100-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/10/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215151 11/10/2023 VOQUEZNA
vonoprazan fumarate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:81520-200 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength vonoprazan fumarate (UNII: 4QW3X4AMLB) (vonoprazan - UNII:1R5L3J156G) vonoprazan fumarate 26.72 mg Inactive Ingredients Ingredient Name Strength Mannitol (UNII: 3OWL53L36A) Microcrystalline Cellulose (UNII: OP1R32D61U) Hydroxypropyl Cellulose, Unspecified (UNII: 9XZ8H6N6OH) Fumaric Acid (UNII: 88XHZ13131) Ascorbic acid (UNII: PQ6CK8PD0R) Croscarmellose sodium (UNII: M28OL1HH48) Magnesium Stearate (UNII: 70097M6I30) Hypromellose, unspecified (UNII: 3NXW29V3WO) Polyethylene Glycol 8000 (UNII: Q662QK8M3B) Titanium Dioxide (UNII: 15FIX9V2JP) Ferric Oxide Red (UNII: 1K09F3G675) Product Characteristics Color RED (pale red) Score no score Shape OVAL Size 11mm Flavor Imprint Code V20 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:81520-200-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/10/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215151 11/10/2023 Labeler - Phathom Pharmaceuticals Inc. (117232216)




