Label: ALUMINUM HYDROXIDE, MAGNESIUM HYDROXIDE AND SIMETHICONE SUSPENSION MAXIMUM STRENGTH- aluminum hydroxide, magnesium hydroxide, and simethicone suspension
ALUMINUM HYDROXIDE, MAGNESIUM HYDROXIDE AND SIMETHICONE SUSPENSION- aluminum hydroxide, magnesium hydroxide, and simethicone suspension
- NDC Code(s): 0904-7325-62, 0904-7325-73, 0904-7326-62, 0904-7326-73
- Packager: MAJOR® PHARMACEUTICALS
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated August 1, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
-
ACTIVE INGREDIENT
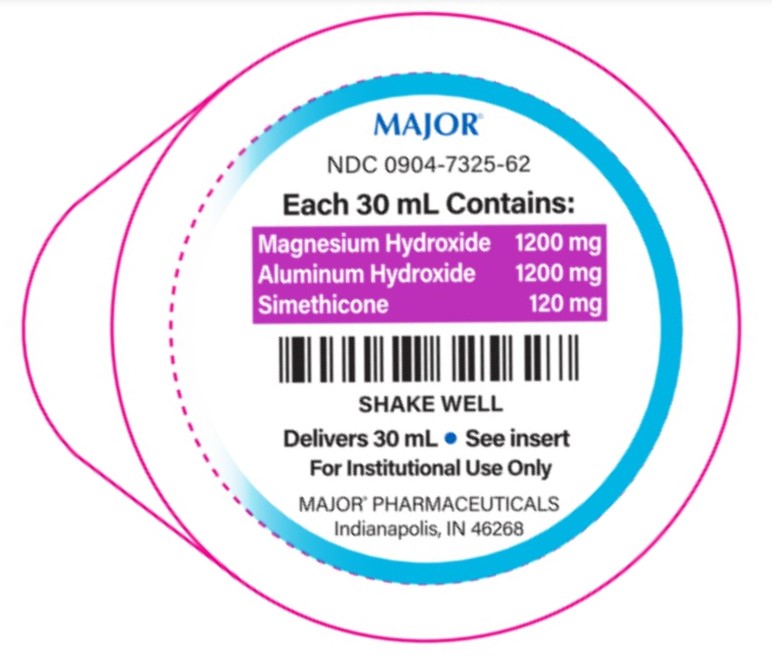
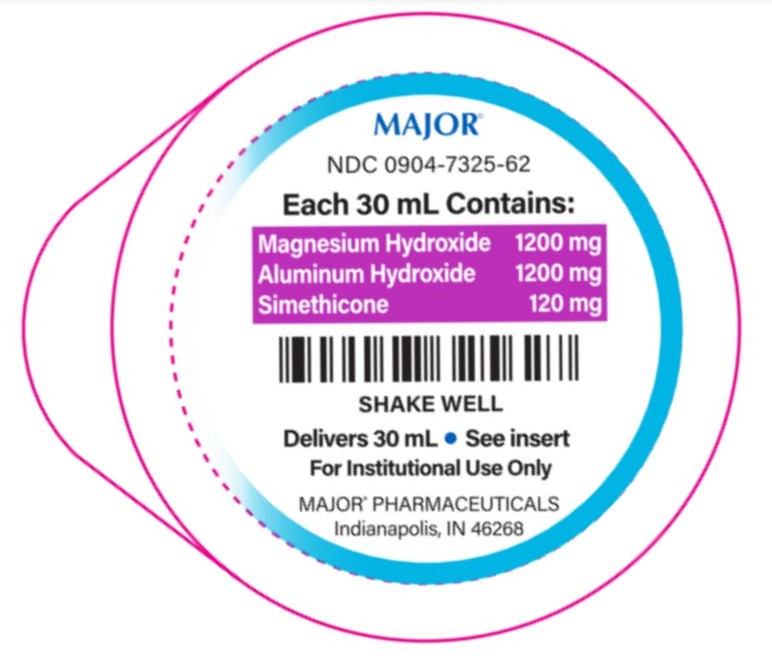
1,200-1,200-120 mg/30 mL
Active ingredients (in each 30 mL) Purpose
Aluminum hydroxide 1,200 mg (equivalent to dried gel, USP)..............Antacid
Magnesium hydroxide 1,200 mg......................................................Antacid
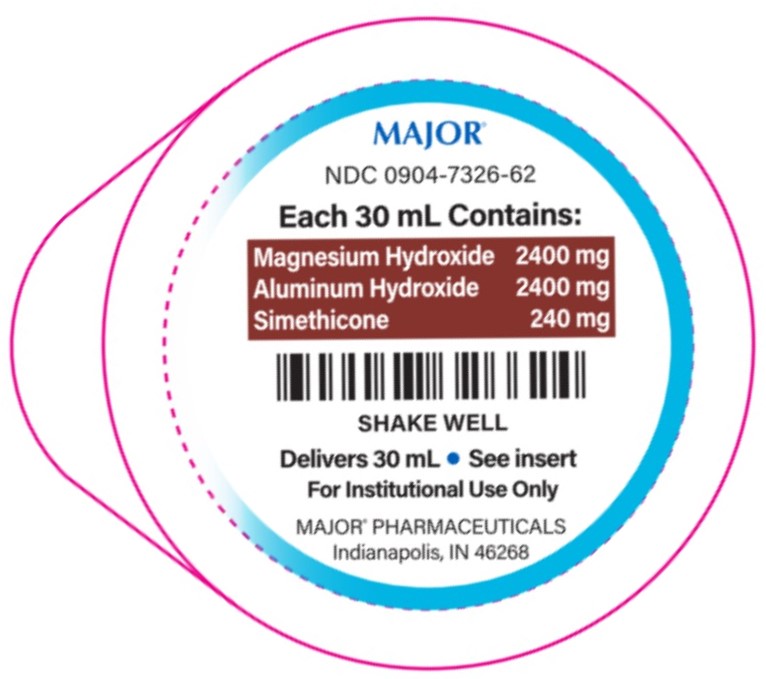
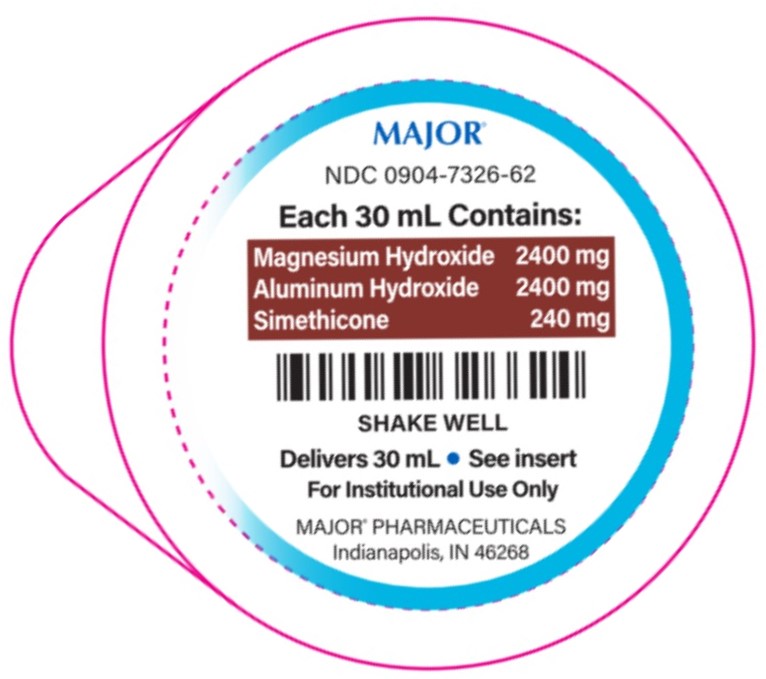
Simethicone 120 mg......................................................................Antigas2,400-2,400-240 mg/30 mL
Active ingredient (in each 30 mL) Purpose
Aluminum hydroxide 2,400 mg (equivalent to dried gel, USP)..............Antacid
Magnesium hydroxide 2,400 mg......................................................Antacid
Simethicone 240 mg......................................................................Antigas - Warnings
-
Directions • shake well before use
1,200-1,200-120 mg/30 mL
age dose Adults and children 12 years and older 30mL, not more than 120 mL in 24 hrs Children under 6 years of age Do not use unless directed by a doctor 2,400-2,400-240 mg/30 mL
age dose Adults and children 12 years and older 30mL, not more than 60 mL in 24 hrs Children under 6 years of age Do not use unless directed by a doctor -
Other information
1,200-1,200-120 mg/30 mL
- each 30 mL containsmagnesium 510 mg and sodium 18 mg
- protect from freezing
- store at 20° to 25°C (68° to 77°F).
- do not use if lid seal is open or damaged
- retain this insert for full product information
2,400-2,400-240 mg/30 mL
- each 30 mL containsmagnesium 990 mg, sodium 30 mg
- store at 20° to 25°C (68° to 77°F).
- protect from freezing
- do not use if lid seal is open or damaged
- retain this insert for full product information
- INACTIVE INGREDIENT
- QUESTIONS
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALUMINUM HYDROXIDE, MAGNESIUM HYDROXIDE AND SIMETHICONE SUSPENSION MAXIMUM STRENGTH
aluminum hydroxide, magnesium hydroxide, and simethicone suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-7326 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 240 mg in 30 mL ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 2400 mg in 30 mL MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838, HYDROXIDE ION - UNII:9159UV381P) MAGNESIUM HYDROXIDE 2400 mg in 30 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYLPARABEN (UNII: 3QPI1U3FV8) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) HYPROMELLOSES (UNII: 3NXW29V3WO) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL SOLUTION (UNII: 8KW3E207O2) Product Characteristics Color Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-7326-73 10 in 1 CASE 08/01/2023 1 10 in 1 TRAY 1 NDC:0904-7326-62 30 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 08/01/2023 ALUMINUM HYDROXIDE, MAGNESIUM HYDROXIDE AND SIMETHICONE SUSPENSION
aluminum hydroxide, magnesium hydroxide, and simethicone suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:0904-7325 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 1200 mg in 30 mL MAGNESIUM HYDROXIDE (UNII: NBZ3QY004S) (MAGNESIUM CATION - UNII:T6V3LHY838, HYDROXIDE ION - UNII:9159UV381P) MAGNESIUM HYDROXIDE 1200 mg in 30 mL DIMETHICONE (UNII: 92RU3N3Y1O) (DIMETHICONE - UNII:92RU3N3Y1O) DIMETHICONE 120 mg in 30 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYLPARABEN (UNII: 3QPI1U3FV8) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SORBITOL SOLUTION (UNII: 8KW3E207O2) Product Characteristics Color Score Shape Size Flavor MINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:0904-7325-73 10 in 1 CASE 08/01/2023 1 10 in 1 TRAY 1 NDC:0904-7325-62 30 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 07/05/2023 Labeler - MAJOR® PHARMACEUTICALS (191427277)