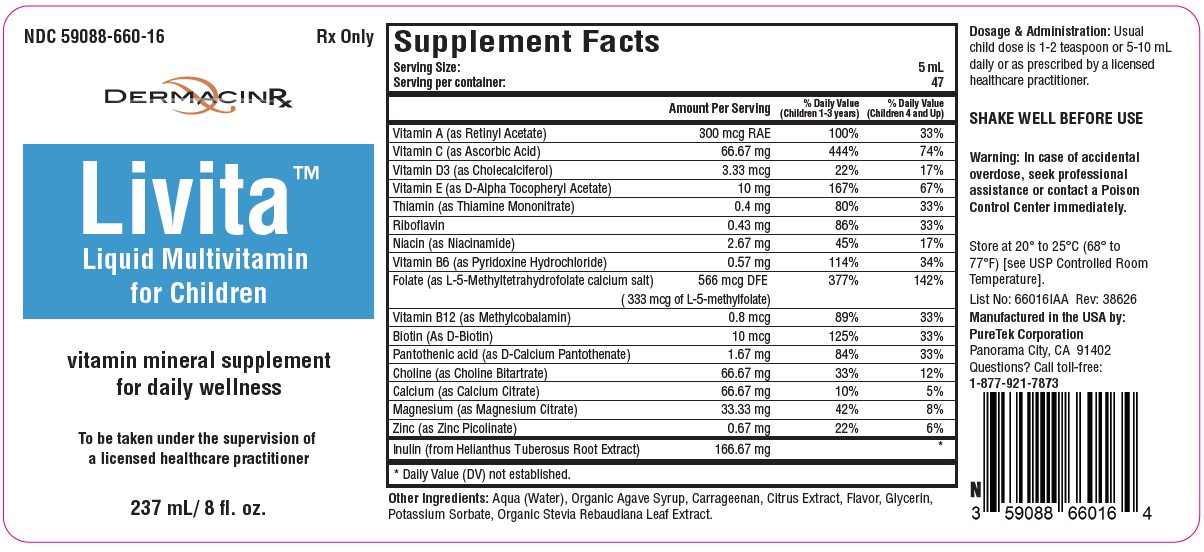
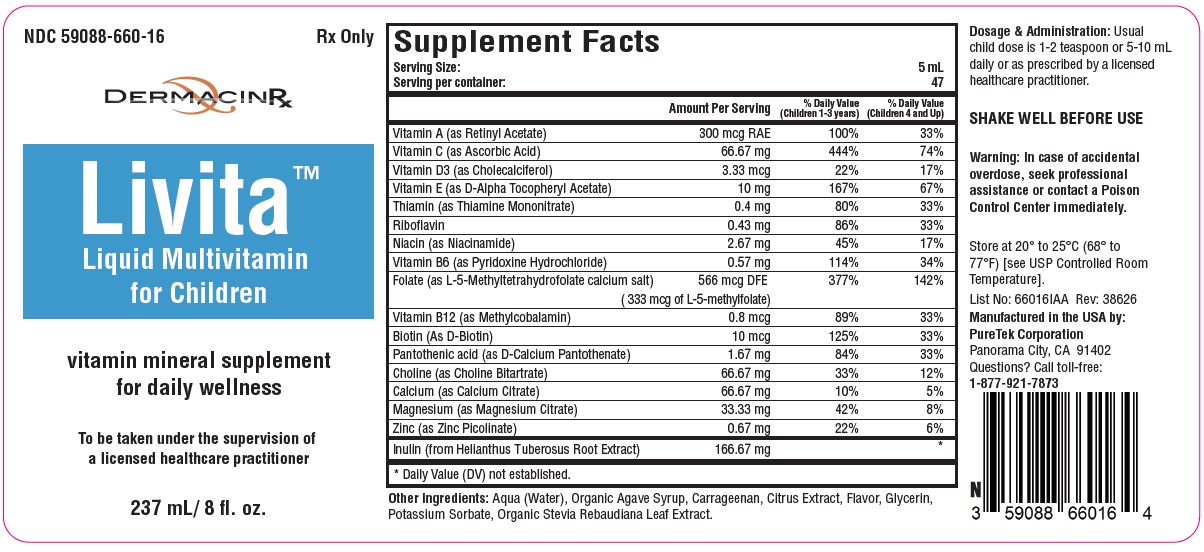
Label: LIVITA CHILDREN- folate, multivitamin liquid
- NDC Code(s): 59088-660-16
- Packager: PureTek Corporation
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved drug other
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 18, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
DESCRIPTION:
Serving Size: 15 mL
Servings per container: 47Vitamin A (as Retinyl Acetate)........................ 300 mcg RAE
Vitamin C (as Ascorbic Acid)............................... 66.67 mg
Vitamin D3 (as Cholecalciferol)............................ 3.33 mcg
Vitamin E (as D-Alpha Tocopheryl Acetate)............. 10 mg
Thiamin (as Thiamine Mononitrate)......................... 0.4 mg
Riboflavin .............................................................. 0.43 mg
Niacin (as Niacinamide)......................................... 2.67 mg
Vitamin B6 (as Pyridoxine Hydrochloride)............. 0.57 mg
Folate (as L-5-Methyltetrahydrofolate calcium salt)...566 mcg DFE
(333 mcg of L-5-Methylfolate)
Vitamin B12 (as Methylcobalamin)......................... 0.8 mcg
Biotin (as D-Biotin)................................................. 10 mcg
Pantothenic Acid (as D-Calcium Pantothenate)..... 1.67 mg
Choline (as Choline Bitartrate)............................. 66.67 mg
Calcium (as Calcium Citrate)............................... 66.67 mg
Magnesium (as Magnesium Citrate)...................... 33.3 mg
Zinc (as Zinc Picolinate)........................................ 0.67 mg
Inulin (as Helianthus Tuberosus Root Extract)... 166.67 mgOther Ingredients: Aqua (Water), Organic Agave Syrup, Carrageenan, Citrus Extract, Flavor, Glycerin, Potassium Sorbate, Organic Stevia Rebaudiana Leaf Extract.
-
INDICATIONS AND USAGE:
Livita™ liquid is indicated to provide significant amounts of essential vitamins and minerals, including Vitamins A, C, D, E, thiamine, riboflavin, niacin, vitamin B6, vitamin B12, folate, calcium, magnesium, and zinc, to supplement the diet. This comprehensive nutrient profile helps prevent nutritional deficiencies of these vitamins and minerals, ensuring that the specific dietary needs of adults and children are met to support overall health, energy, and vitality. The product is specially formulated to target common vitamin and mineral gaps in adults, thus promoting optimal health, immune function, bone strength, and metabolic balance. It is intended to be used under the guidance of a licensed healthcare practitioner to ensure that any potential for nutritional deficiency is addressed in a manner that supports the individual's overall health and wellbeing.
- Contraindications:
-
WARNING:
In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
Precaution Section
Folate doses above 0.1 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive. There is a potential danger in administering folate to patients with undiagnosed anemia since folate may obscure the diagnosis of pernicious anemia by alleviating the hematologic manifestations of the disease while allowing the neurologic complications to progress. This may result in severe nervous system damage before the correct diagnosis is made. Adequate doses of vitamin B12 may prevent, halt, or improve the neurologic changes caused by pernicious anemia.
The patient’s medical conditions and consumption of other drugs, herbs, and/or supplements should be considered.
For use on the order of a licensed healthcare practitioner. Call your doctor about side effects. To report side effects, call PureTek Corporation at 1-877-921-7873 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Adverse Reactions:
Folate: Allergic sensitizations have been reported following both oral and parenteral administration of folate. Adverse reactions have been reported with specific vitamins and minerals but generally at levels substantially higher than those contained herein. However, allergic, and idiosyncratic reactions are possible at lower levels.
- Dosage and Administration:
- How Supplied:
- STORAGE:
- Livita™
-
INGREDIENTS AND APPEARANCE
LIVITA CHILDREN
folate, multivitamin liquidProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:59088-660 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHOLINE BITARTRATE (UNII: 6K2W7T9V6Y) (CHOLINE - UNII:N91BDP6H0X) CHOLINE 66.67 mg in 1 mL CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 3.33 ug in 1 mL VITAMIN A ACETATE (UNII: 3LE3D9D6OY) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 300 ug in 1 mL ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 66.67 mg in 1 mL .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 10 mg in 1 mL THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 0.4 mg in 1 mL RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 0.43 mg in 1 mL NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 2.67 mg in 1 mL PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 0.57 mg in 1 mL LEVOMEFOLATE CALCIUM (UNII: A9R10K3F2F) (LEVOMEFOLIC ACID - UNII:8S95DH25XC) LEVOMEFOLATE CALCIUM 333 ug in 1 mL METHYLCOBALAMIN (UNII: BR1SN1JS2W) (METHYLCOBALAMIN - UNII:BR1SN1JS2W) METHYLCOBALAMIN 0.8 ug in 1 mL BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 10 ug in 1 mL ZINC PICOLINATE (UNII: ALO92O31SE) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 0.67 mg in 1 mL MAGNESIUM CITRATE (UNII: RHO26O1T9V) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM CITRATE 33.33 mg in 1 mL CALCIUM PANTOTHENATE (UNII: 568ET80C3D) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 1.67 mg in 1 mL CALCIUM CITRATE (UNII: MLM29U2X85) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CITRATE 66.67 mg in 1 mL INULIN (UNII: JOS53KRJ01) (INULIN - UNII:JOS53KRJ01) INULIN 166.67 mg in 1 mL Inactive Ingredients Ingredient Name Strength AGAVE TEQUILANA JUICE (UNII: GVG8G0207O) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) CARRAGEENAN (UNII: 5C69YCD2YJ) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) STEVIA REBAUDIUNA LEAF (UNII: 6TC6NN0876) CITRUS FRUIT (UNII: XDK00Z8012) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59088-660-16 237 mL in 1 BOTTLE; Type 0: Not a Combination Product 12/18/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 12/18/2023 Labeler - PureTek Corporation (785961046)