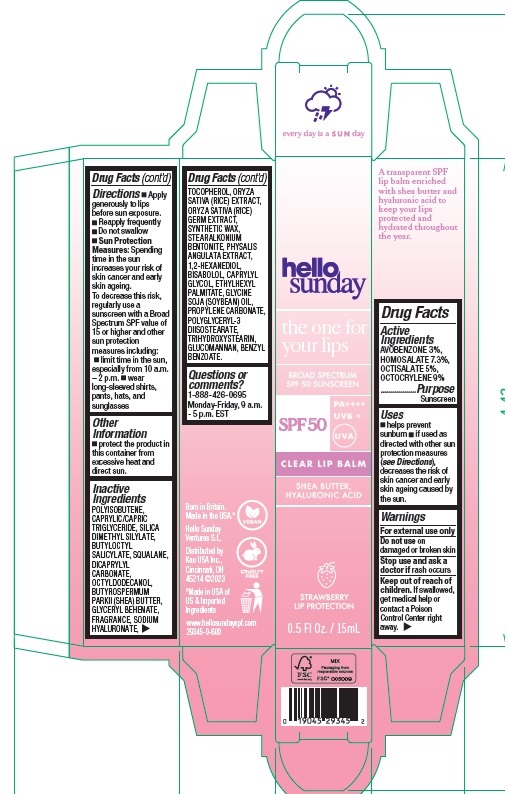
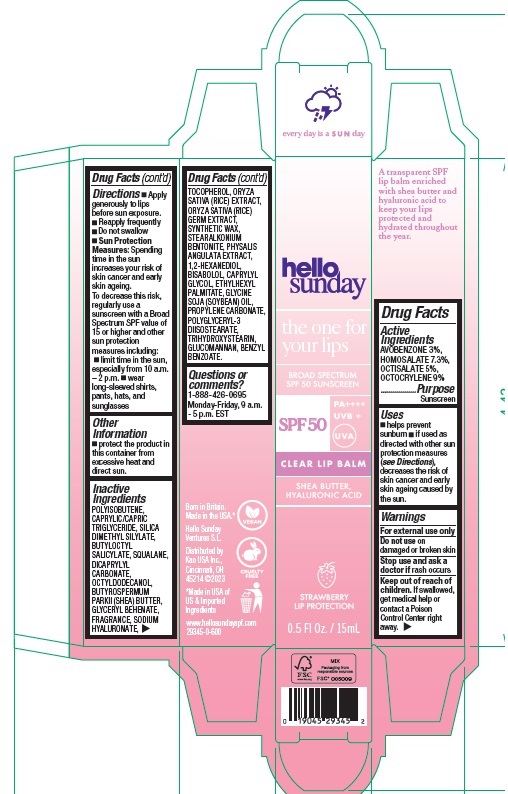
Label: HELLO SUNDAY THE ONE FOR YOUR LIPS CLEAR LIP BALM SPF 50- avobenzone, homosalate, octisalate, octocrylene stick
- NDC Code(s): 10596-806-01
- Packager: Kao USA Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated December 15, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Purpose
- Uses
- Warnings
-
Directions
- Apply generously to lips before sun exposure.
- Reapply frequently
- Do not swallow
- Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin ageing. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- Other Information
-
Inactive Ingredients
POLYISOBUTENE, CAPRYLIC/CAPRIC TRIGLYCERIDE, SILICA DIMETHYL SILYLATE, BUTYLOCTYL SALICYLATE, SQUALANE, DICAPRYLYL CARBONATE, OCTYLDODECANOL, BUTYROSPERMUM PARKII (SHEA) BUTTER, GLYCERYL BEHENATE, FRAGRANCE, SODIUM HYALURONATE, TOCOPHEROL, ORYZA SATIVA (RICE) EXTRACT, ORYZA SATIVA (RICE) GERM EXTRACT, SYNTHETIC WAX, STEARALKONIUM BENTONITE, PHYSALIS ANGULATA EXTRACT, 1,2-HEXANEDIOL, BISABOLOL, CAPRYLYL GLYCOL, ETHYLHEXYL PALMITATE, GLYCINE SOJA (SOYBEAN) OIL, PROPYLENE CARBONATE, POLYGLYCERYL-3 DIISOSTEARATE, TRIHYDROXYSTEARIN, GLUCOMANNAN, BENZYL BENZOATE.
- Questions or comments?
- Product Packaging
-
INGREDIENTS AND APPEARANCE
HELLO SUNDAY THE ONE FOR YOUR LIPS CLEAR LIP BALM SPF 50
avobenzone, homosalate, octisalate, octocrylene stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10596-806 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 50 mg in 1 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 90 mg in 1 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 73 mg in 1 mL Inactive Ingredients Ingredient Name Strength POLYISOBUTYLENE (1300 MW) (UNII: 241BN7J12Y) HYALURONATE SODIUM (UNII: YSE9PPT4TH) RICE GERM (UNII: 7N2B70SFEZ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TRIHYDROXYSTEARIN (UNII: 06YD7896S3) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) SQUALANE (UNII: GW89575KF9) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) OCTYLDODECANOL (UNII: 461N1O614Y) SHEA BUTTER (UNII: K49155WL9Y) TOCOPHEROL (UNII: R0ZB2556P8) GLYCERYL MONOBEHENATE (UNII: A626UU0W2A) SYNTHETIC WAX (1200 MW) (UNII: Q3Z4BCH099) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) PROPYLENE CARBONATE (UNII: 8D08K3S51E) ETHYLHEXYL PALMITATE (UNII: 2865993309) LEVOMENOL (UNII: 24WE03BX2T) KONJAC MANNAN (UNII: 36W3E5TAMG) PHYSALIS ANGULATA WHOLE (UNII: W4TKW9D5GG) CAPRYLYL GLYCOL (UNII: 00YIU5438U) BENZYL BENZOATE (UNII: N863NB338G) POLYGLYCERYL-3 DIISOSTEARATE (UNII: 46P231IQV8) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SOYBEAN OIL (UNII: 241ATL177A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10596-806-01 1 in 1 CARTON 02/01/2024 1 15 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 02/01/2024 Labeler - Kao USA Inc. (004251617)