Label: DUKAL WHITE PETROLATUM- petrolatum jelly
- NDC Code(s): 65517-2023-1
- Packager: Dukal LLC
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
Drug Label Information
Updated January 8, 2024
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
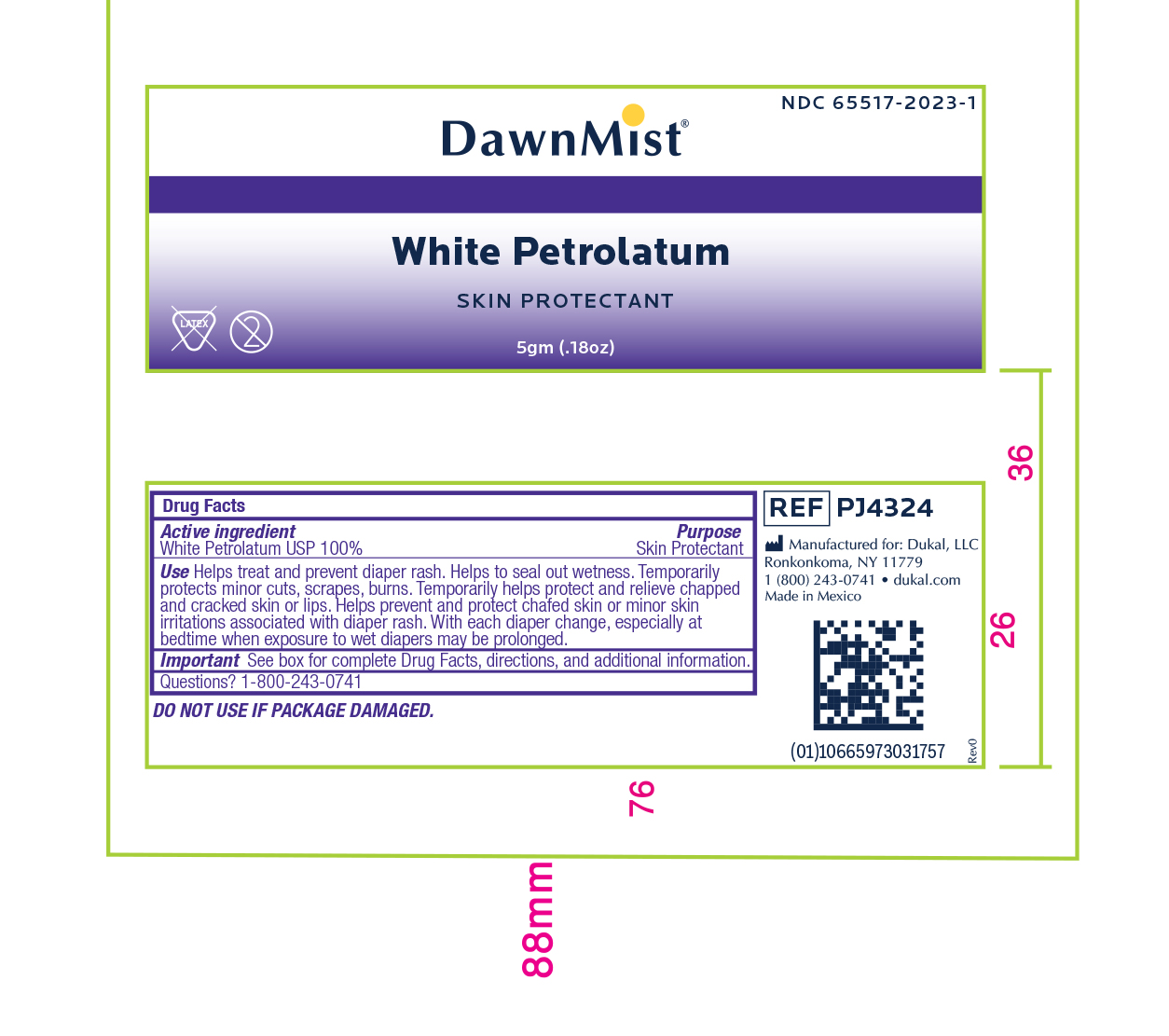
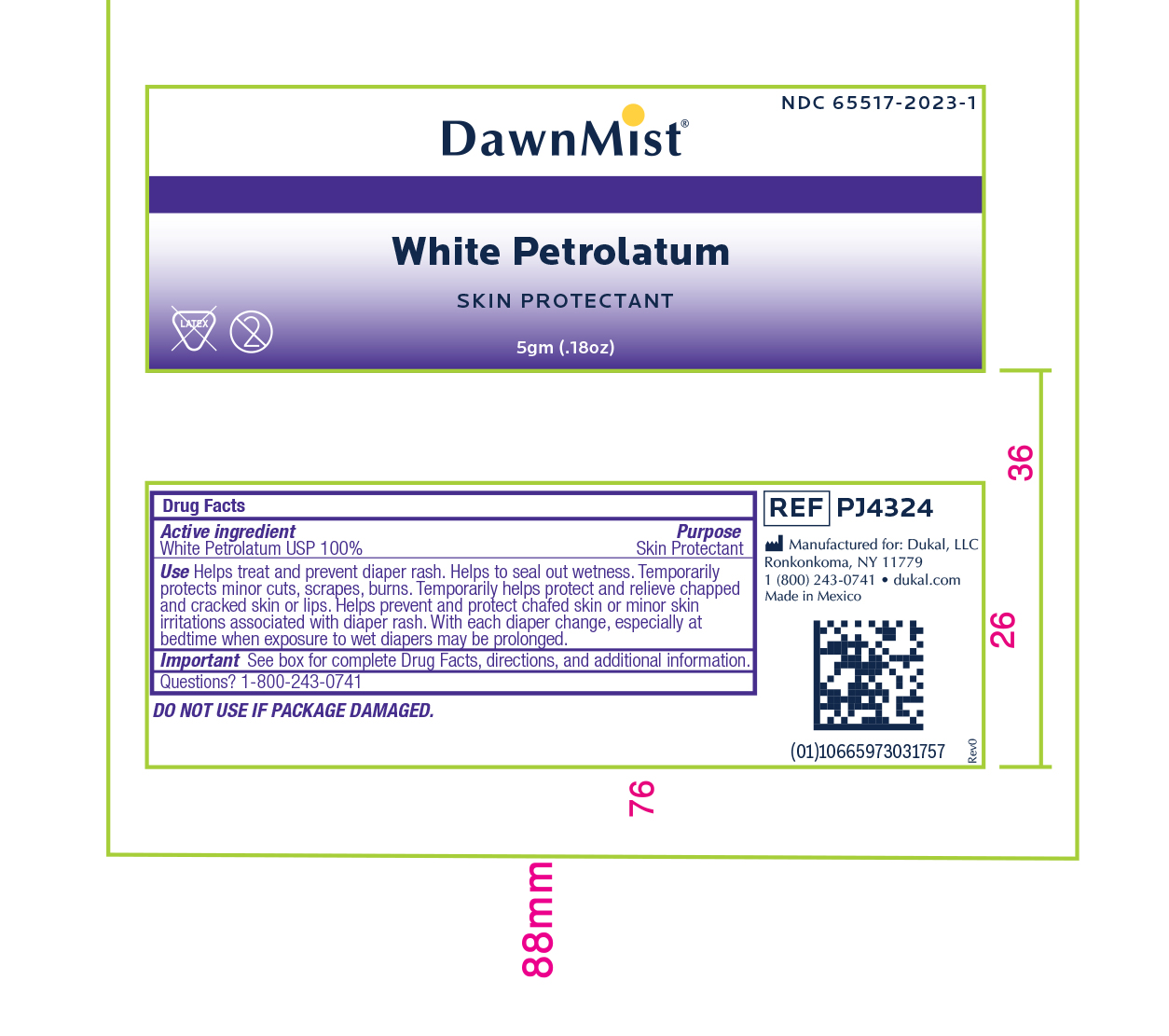
- Active Ingredient
- Purpose
-
Uses:
- Helps treat and prevent diaper rash
- Helps seal out wetness
- Temporarily protects minor * cuts * scrapes * burns
- Temporarily helps protect and relieve chapped and cracked skin or lips
- Helps prevent and protect chafed skin or minor skin irritations associated with diaper rash
- With each diaper change, especially at bedtime when exposure to wet diapers may be prolonged.
- Warnings:
- Directions:
- Other Information
- Inactive Ingredients
- Principal Display Panel
-
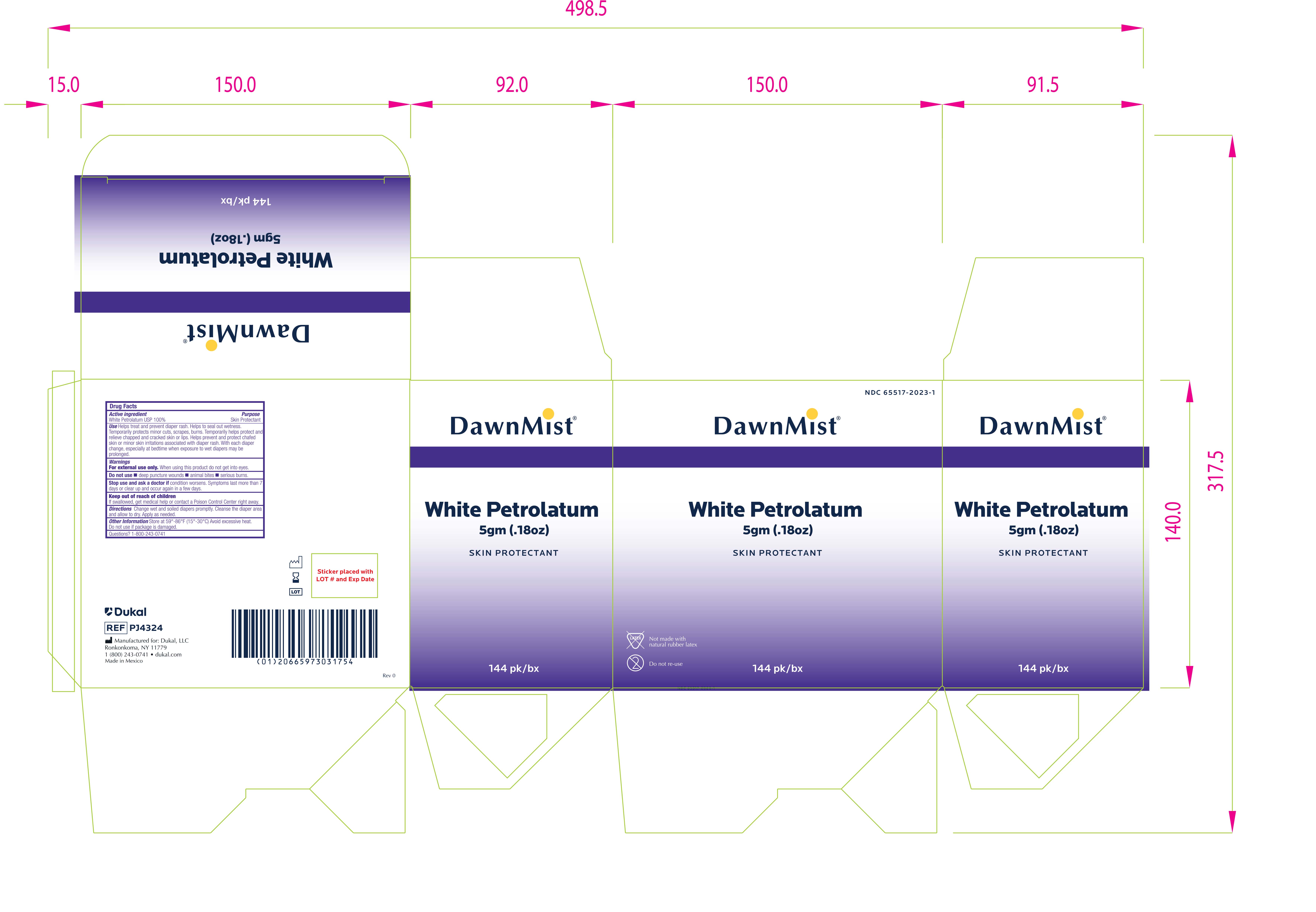
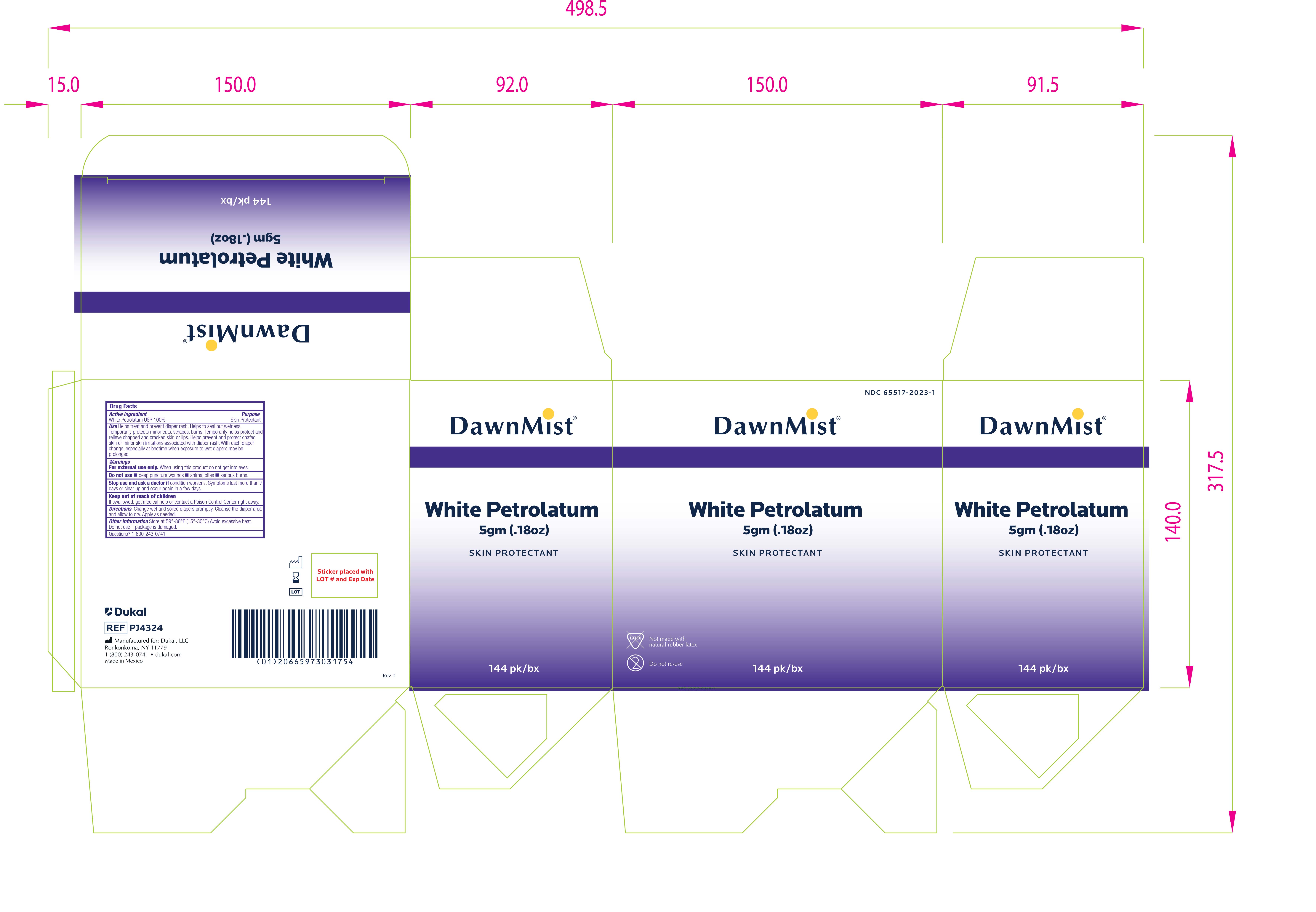
INGREDIENTS AND APPEARANCE
DUKAL WHITE PETROLATUM
petrolatum jellyProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65517-2023 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 1 g in 1 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65517-2023-1 6 in 1 CASE 12/13/2023 1 144 in 1 BOX 1 5 g in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 12/13/2023 Labeler - Dukal LLC (791014871)