Label: ETODOLAC tablet, film coated, extended release
-
NDC Code(s):
65841-777-01,
65841-777-05,
65841-777-10,
65841-777-14, view more65841-777-30, 65841-777-77, 65841-778-01, 65841-778-05, 65841-778-10, 65841-778-14, 65841-778-30, 65841-778-77, 65841-779-01, 65841-779-05, 65841-779-10, 65841-779-14, 65841-779-30, 65841-779-77
- Packager: Zydus Lifesciences Limited
- Category: HUMAN PRESCRIPTION DRUG LABEL
- DEA Schedule: None
- Marketing Status: Abbreviated New Drug Application
Drug Label Information
Updated September 26, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- SPL MEDGUIDE
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 65841-777-14 in bottle of 60 tablets
Etodolac Extended-release Tablets USP, 400 mg
Rx only
60 tablets

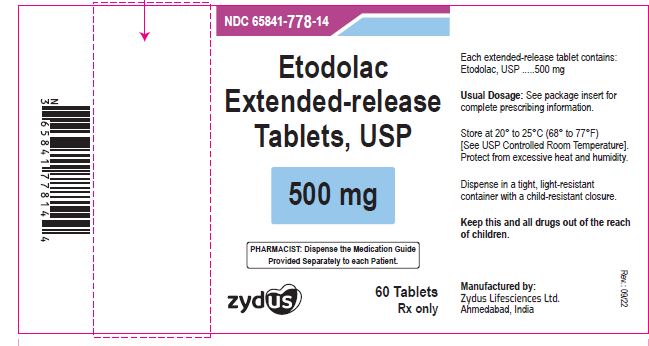
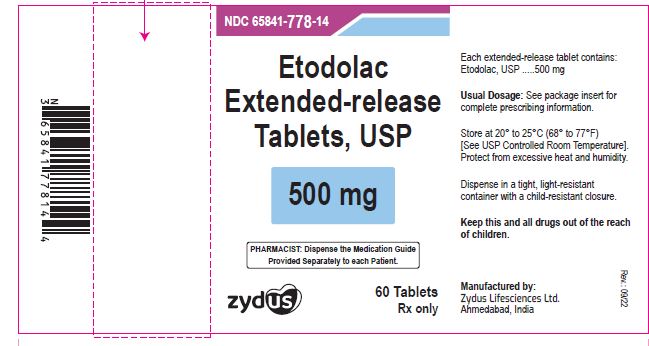
NDC 65841-778-14 in bottle of 60 tablets
Etodolac Extended-release Tablets USP, 500 mg
Rx only
60 tablets
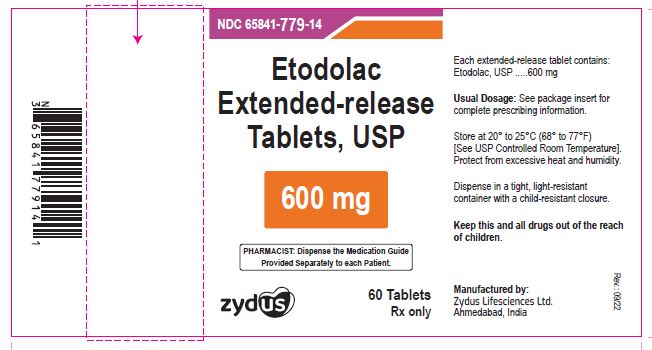
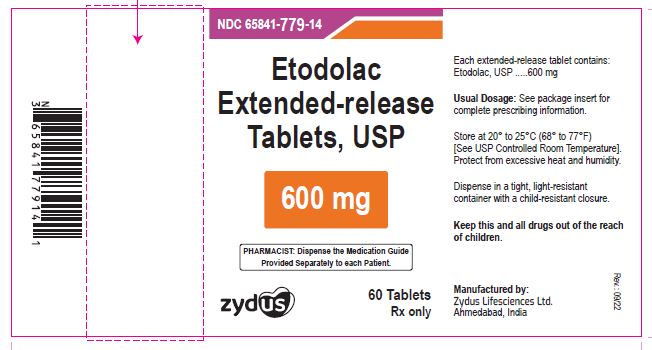
NDC 65841-779-14 in bottle of 60 tablets
Etodolac Extended-release Tablets USP, 600 mg
Rx only
60 tablets
-
INGREDIENTS AND APPEARANCE
ETODOLAC
etodolac tablet, film coated, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-777 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ETODOLAC (UNII: 2M36281008) (ETODOLAC - UNII:2M36281008) ETODOLAC 400 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) ETHYLCELLULOSE (100 MPA.S) (UNII: 47MLB0F1MV) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) Product Characteristics Color ORANGE (ORANGE) Score no score Shape OVAL (OVAL) Size 17mm Flavor Imprint Code 271 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-777-14 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2014 2 NDC:65841-777-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2014 3 NDC:65841-777-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2014 4 NDC:65841-777-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2014 5 NDC:65841-777-77 100 in 1 CARTON 02/15/2014 5 NDC:65841-777-30 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091134 02/15/2014 ETODOLAC
etodolac tablet, film coated, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-778 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ETODOLAC (UNII: 2M36281008) (ETODOLAC - UNII:2M36281008) ETODOLAC 500 mg Inactive Ingredients Ingredient Name Strength ETHYLCELLULOSE (100 MPA.S) (UNII: 47MLB0F1MV) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) Product Characteristics Color GRAY (GRAY) Score no score Shape OVAL (OVAL) Size 18mm Flavor Imprint Code 272 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-778-14 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2014 2 NDC:65841-778-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2014 3 NDC:65841-778-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2014 4 NDC:65841-778-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2014 5 NDC:65841-778-77 100 in 1 CARTON 02/15/2014 5 NDC:65841-778-30 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091134 02/15/2014 ETODOLAC
etodolac tablet, film coated, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:65841-779 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ETODOLAC (UNII: 2M36281008) (ETODOLAC - UNII:2M36281008) ETODOLAC 600 mg Inactive Ingredients Ingredient Name Strength ETHYLCELLULOSE (100 MPA.S) (UNII: 47MLB0F1MV) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) HYPROMELLOSES (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) SODIUM PHOSPHATE, DIBASIC, ANHYDROUS (UNII: 22ADO53M6F) Product Characteristics Color BLUE (BLUE) Score no score Shape OVAL (OVAL) Size 19mm Flavor Imprint Code 273 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65841-779-14 60 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2014 2 NDC:65841-779-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2014 3 NDC:65841-779-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2014 4 NDC:65841-779-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2014 5 NDC:65841-779-77 100 in 1 CARTON 02/15/2014 5 NDC:65841-779-30 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091134 02/15/2014 Labeler - Zydus Lifesciences Limited (918596198) Registrant - Zydus Lifesciences Limited (918596198) Establishment Name Address ID/FEI Business Operations Zydus Lifesciences Limited 918596198 ANALYSIS(65841-777, 65841-778, 65841-779) , MANUFACTURE(65841-777, 65841-778, 65841-779)