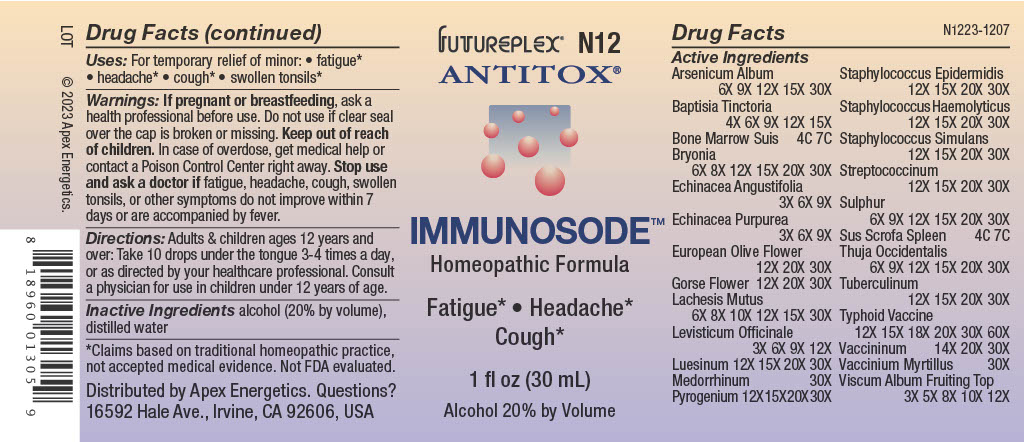
Label: N12 IMMUNOSODE- arsenic trioxide, baptisia tinctoria root, sus scrofa bone marrow, bryonia alba root, echinacea angustifolia, echinacea purpurea, olea europaea flower, ulex europaeus, flower, lachesis muta venom, levisticum officinale, treponemic skin canker human solution/ drops
- NDC Code(s): 63479-1412-2
- Packager: Apex Energetics Inc.
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 29, 2023
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
Active Ingredients
Arsenicum Album
6X 9X 12X 15X 30X
Baptisia Tinctoria
4X 6X 9X 12X 15X
Bone Marrow Suis
4C 7C
Bryonia
6X 8X 12X 15X 20X 30X
Echinacea Angustifolia
3X 6X 9X
Echinacea Purpurea
3X 6X 9X
European Olive Flower
12X 20X 30X
Gorse Flower
12X 20X 30X
Lachesis Mutus
6X 8X 10X 12X 15X 30X
Levisticum Officinale
3X 6X 9X 12X
Luesinum
12X 15X 20X 30X
Medorrhinum
30X
Pyrogenium
12X 15X 20X 30X
Staphylococcus Epidermidis
12X 15X 20X 30X
Staphylococcus Haemolyticus
12X 15X 20X 30X
Staphylococcus Simulans
12X 15X 20X 30X
Streptococcinum
12X 15X 20X 30X
Sulphur
6X 9X 12X 15X 20X 30X
Sus Scrofa Spleen
4C 7C
Thuja Occidentalis
6X 9X 12X 15X 20X 30X
Tuberculinum
12X 15X 20X 30X
Typhoid Vaccine
12X 15X 18X 20X 30X 60X
Vaccininum
14X 20X 30X
Vaccinium Myrtillus
30X
Viscum Album Fruiting Top
3X 5X 8X 10X 12X
- INDICATIONS & USAGE
- Warnings:
- Directions:
- Inactive Ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
N12 IMMUNOSODE
arsenic trioxide, baptisia tinctoria root, sus scrofa bone marrow, bryonia alba root, echinacea angustifolia, echinacea purpurea, olea europaea flower, ulex europaeus, flower, lachesis muta venom, levisticum officinale, treponemic skin canker human solution/ dropsProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63479-1412 Route of Administration SUBLINGUAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VISCUM ALBUM FRUITING TOP (UNII: BK9092J5MP) (VISCUM ALBUM FRUITING TOP - UNII:BK9092J5MP) VISCUM ALBUM FRUITING TOP 12 [hp_X] in 1 mL GONORRHEAL URETHRAL SECRETION HUMAN (UNII: 9BZG9E3I8F) (GONORRHEAL URETHRAL SECRETION HUMAN - UNII:9BZG9E3I8F) GONORRHEAL URETHRAL SECRETION HUMAN 30 [hp_X] in 1 mL RANCID BEEF (UNII: 29SUH5R3HU) (RANCID BEEF - UNII:29SUH5R3HU) RANCID BEEF 30 [hp_X] in 1 mL STREPTOCOCCUS PYOGENES (UNII: LJ2LP0YL98) (STREPTOCOCCUS PYOGENES - UNII:LJ2LP0YL98) STREPTOCOCCUS PYOGENES 30 [hp_X] in 1 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 30 [hp_X] in 1 mL SUS SCROFA SPLEEN (UNII: 92AMN5J79Y) (SUS SCROFA SPLEEN - UNII:92AMN5J79Y) SUS SCROFA SPLEEN 7 [hp_C] in 1 mL BILBERRY (UNII: 9P2U39H18W) (BILBERRY - UNII:9P2U39H18W) BILBERRY 30 [hp_X] in 1 mL STAPHYLOCOCCUS EPIDERMIDIS (UNII: D5T403TNGE) (STAPHYLOCOCCUS EPIDERMIDIS - UNII:D5T403TNGE) STAPHYLOCOCCUS EPIDERMIDIS 30 [hp_X] in 1 mL ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 30 [hp_X] in 1 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA WHOLE - UNII:QI7G114Y98) ECHINACEA PURPUREA 9 [hp_X] in 1 mL OLEA EUROPAEA FLOWER (UNII: 498M34P1VZ) (OLEA EUROPAEA FLOWER - UNII:498M34P1VZ) OLEA EUROPAEA FLOWER 30 [hp_X] in 1 mL TUBERCULIN PURIFIED PROTEIN DERIVATIVE (UNII: I7L8FKN87J) (TUBERCULIN PURIFIED PROTEIN DERIVATIVE - UNII:I7L8FKN87J) TUBERCULIN PURIFIED PROTEIN DERIVATIVE 30 [hp_X] in 1 mL SALMONELLA ENTERICA ENTERICA SEROVAR TYPHI (UNII: 760T5R8B3O) (SALMONELLA ENTERICA ENTERICA SEROVAR TYPHI - UNII:760T5R8B3O) SALMONELLA ENTERICA ENTERICA SEROVAR TYPHI 60 [hp_X] in 1 mL VACCINIA VIRUS STRAIN NEW YORK CITY BOARD OF HEALTH LIVE ANTIGEN (UNII: 4SV59689SK) (VACCINIA VIRUS STRAIN NEW YORK CITY BOARD OF HEALTH LIVE ANTIGEN - UNII:4SV59689SK) VACCINIA VIRUS STRAIN NEW YORK CITY BOARD OF HEALTH LIVE ANTIGEN 30 [hp_X] in 1 mL SUS SCROFA BONE MARROW (UNII: VP2CN2G7Y8) (SUS SCROFA BONE MARROW - UNII:VP2CN2G7Y8) SUS SCROFA BONE MARROW 7 [hp_C] in 1 mL TREPONEMIC SKIN CANKER HUMAN (UNII: 4ZWP7FWI8W) (TREPONEMIC SKIN CANKER HUMAN - UNII:4ZWP7FWI8W) TREPONEMIC SKIN CANKER HUMAN 30 [hp_X] in 1 mL THUJA OCCIDENTALIS LEAFY TWIG (UNII: 1NT28V9397) (THUJA OCCIDENTALIS LEAFY TWIG - UNII:1NT28V9397) THUJA OCCIDENTALIS LEAFY TWIG 30 [hp_X] in 1 mL BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 15 [hp_X] in 1 mL ECHINACEA ANGUSTIFOLIA (UNII: VB06AV5US8) (ECHINACEA ANGUSTIFOLIA WHOLE - UNII:VB06AV5US8) ECHINACEA ANGUSTIFOLIA 9 [hp_X] in 1 mL LEVISTICUM OFFICINALE (UNII: SZD8739PH1) (LEVISTICUM OFFICINALE - UNII:SZD8739PH1) LEVISTICUM OFFICINALE 12 [hp_X] in 1 mL STAPHYLOCOCCUS HAEMOLYTICUS (UNII: 092IJ99835) (STAPHYLOCOCCUS HAEMOLYTICUS - UNII:092IJ99835) STAPHYLOCOCCUS HAEMOLYTICUS 30 [hp_X] in 1 mL STAPHYLOCOCCUS SIMULANS (UNII: V68E0X60VL) (STAPHYLOCOCCUS SIMULANS - UNII:V68E0X60VL) STAPHYLOCOCCUS SIMULANS 30 [hp_X] in 1 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 30 [hp_X] in 1 mL ULEX EUROPAEUS FLOWER (UNII: 398DBS1PXN) (ULEX EUROPAEUS FLOWER - UNII:398DBS1PXN) ULEX EUROPAEUS FLOWER 30 [hp_X] in 1 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 30 [hp_X] in 1 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63479-1412-2 30 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 01/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/15/2024 Labeler - Apex Energetics Inc. (195816384)