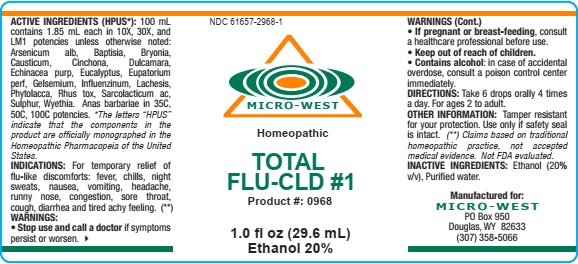
Label: TOTAL FLU CLD 1- arsenicum alb, baptisia, bryonia, causticum, cinchona, dulcamara, echinacea purp, eucalyptus, eupatorium perf, gelsemium, influenzinum, lachesis, phytolacca, rhus tox, sarcolacticum ac, sulphur, wyethia. anas barbariae liquid
- NDC Code(s): 61657-2968-1
- Packager: White Manufacturing Inc. DBA Micro-West
- Category: HUMAN OTC DRUG LABEL
- DEA Schedule: None
- Marketing Status: unapproved homeopathic
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated January 21, 2021
If you are a consumer or patient please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
ACTIVE INGREDIENT
ACTIVE INGREDIENTS (HPUS*): 100 mL
contains 1.85 mL each in 10X, 30X, and
LM1 potencies unless otherwise noted:
Arsenicum alb, Baptisia, Bryonia,
Causticum, Cinchona, Dulcamara,
Echinacea purp, Eucalyptus, Eupatorium
perf, Gelsemium, Influenzinum, Lachesis,
Phytolacca, Rhus tox, Sarcolacticum ac,
Sulphur, Wyethia. Anas barbariae in 35C,
50C, 100C potencies. *The letters “HPUS”
indicate that the components in the
product are officially monographed in the
Homeopathic Pharmacopeia of the United
States - INDICATIONS & USAGE
- WARNINGS
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
TOTAL FLU CLD 1
arsenicum alb, baptisia, bryonia, causticum, cinchona, dulcamara, echinacea purp, eucalyptus, eupatorium perf, gelsemium, influenzinum, lachesis, phytolacca, rhus tox, sarcolacticum ac, sulphur, wyethia. anas barbariae liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61657-2968 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ARSENIC TRIOXIDE (UNII: S7V92P67HO) (ARSENIC CATION (3+) - UNII:C96613F5AV) ARSENIC TRIOXIDE 10 [hp_X] in 30 mL BAPTISIA TINCTORIA ROOT (UNII: 5EF0HWI5WU) (BAPTISIA TINCTORIA ROOT - UNII:5EF0HWI5WU) BAPTISIA TINCTORIA ROOT 10 [hp_X] in 30 mL BRYONIA ALBA ROOT (UNII: T7J046YI2B) (BRYONIA ALBA ROOT - UNII:T7J046YI2B) BRYONIA ALBA ROOT 10 [hp_X] in 30 mL CAUSTICUM (UNII: DD5FO1WKFU) (CAUSTICUM - UNII:DD5FO1WKFU) CAUSTICUM 10 [hp_X] in 30 mL CINCHONA OFFICINALIS BARK (UNII: S003A158SB) (CINCHONA OFFICINALIS BARK - UNII:S003A158SB) CINCHONA OFFICINALIS BARK 10 [hp_X] in 30 mL SOLANUM DULCAMARA TOP (UNII: KPS1B1162N) (SOLANUM DULCAMARA TOP - UNII:KPS1B1162N) SOLANUM DULCAMARA TOP 10 [hp_X] in 30 mL ECHINACEA PURPUREA (UNII: QI7G114Y98) (ECHINACEA PURPUREA - UNII:QI7G114Y98) ECHINACEA PURPUREA 10 [hp_X] in 30 mL EUCALYPTUS GLOBULUS LEAF (UNII: S546YLW6E6) (EUCALYPTUS GLOBULUS LEAF - UNII:S546YLW6E6) EUCALYPTUS GLOBULUS LEAF 10 [hp_X] in 30 mL EUPATORIUM PERFOLIATUM FLOWERING TOP (UNII: 1W0775VX6E) (EUPATORIUM PERFOLIATUM FLOWERING TOP - UNII:1W0775VX6E) EUPATORIUM PERFOLIATUM FLOWERING TOP 10 [hp_X] in 30 mL GELSEMIUM SEMPERVIRENS ROOT (UNII: 639KR60Q1Q) (GELSEMIUM SEMPERVIRENS ROOT - UNII:639KR60Q1Q) GELSEMIUM SEMPERVIRENS ROOT 10 [hp_X] in 30 mL INFLUENZA A VIRUS (UNII: R9HH0NDE2E) (INFLUENZA A VIRUS - UNII:R9HH0NDE2E) INFLUENZA A VIRUS 10 [hp_X] in 30 mL LACHESIS MUTA VENOM (UNII: VSW71SS07I) (LACHESIS MUTA VENOM - UNII:VSW71SS07I) LACHESIS MUTA VENOM 10 [hp_X] in 30 mL PHYTOLACCA AMERICANA ROOT (UNII: 11E6VI8VEG) (PHYTOLACCA AMERICANA ROOT - UNII:11E6VI8VEG) PHYTOLACCA AMERICANA ROOT 10 [hp_X] in 30 mL TOXICODENDRON PUBESCENS LEAF (UNII: 6IO182RP7A) (TOXICODENDRON PUBESCENS LEAF - UNII:6IO182RP7A) TOXICODENDRON PUBESCENS LEAF 10 [hp_X] in 30 mL LACTIC ACID, L- (UNII: F9S9FFU82N) (LACTIC ACID, L- - UNII:F9S9FFU82N) LACTIC ACID, L- 10 [hp_X] in 30 mL SULFUR (UNII: 70FD1KFU70) (SULFUR - UNII:70FD1KFU70) SULFUR 10 [hp_X] in 30 mL WYETHIA HELENIOIDES ROOT (UNII: J10PD1AQ0N) (WYETHIA HELENIOIDES ROOT - UNII:J10PD1AQ0N) WYETHIA HELENIOIDES ROOT 10 [hp_X] in 30 mL CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE (UNII: RN2HC612GY) (CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE - UNII:RN2HC612GY) CAIRINA MOSCHATA HEART/LIVER AUTOLYSATE 35 [hp_C] in 30 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61657-2968-1 30 mL in 1 BOTTLE, DROPPER; Type 0: Not a Combination Product 11/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 11/01/2018 Labeler - White Manufacturing Inc. DBA Micro-West (082307831)