Label: ACETAMINOPHEN,DEXTROMETHORPHAN,DOXYLAMINE capsule, liquid filled
-
Contains inactivated NDC Code(s)
NDC Code(s): 58805-003-04, 58805-003-14 - Packager: Agile Pharmachem
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 4, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
Warnings Failure to follow these warnings could result in serious consequences.
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4 doses in 24 hours which is maximum daily amount for this product
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks daily while using this product
do not use:
- with any other drug containing acetaminophen (prescription or not prescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (Certain drugs for depression, psychiatric or emotional conditions or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug, If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- liver disease
- Heart disease
- Thyroid disease
- Diabetes
- High blood pressure
- Trouble urinating due to enlarged prostate gland
-
ask your doctor or pharmacist before use if you are taking the blood thinning drug warfarin. When using this product, do not use more than directed.
-
Stop use and ask a doctor if:
- Redness or swelling is present
- You get nervous, dizzy or sleepless
- Fever gets worse or lasts more than 3 days
- New symptoms occur
- Symptoms do not get better within 7 days or are accompanied by a fever
-
Keep out of reach of children.
If pregnant or breast-feeding, ask a health professional before use.
Overdose warning: Taking more than recommended dose (overdose) may cause liver damage. In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Tamper evident: this package is safety sealed and child resistant. Use only if blisters are intact. If difficult to open use scissors.
- Direction
- Other Information
- Inactive Ingredients
- Questions or Comments
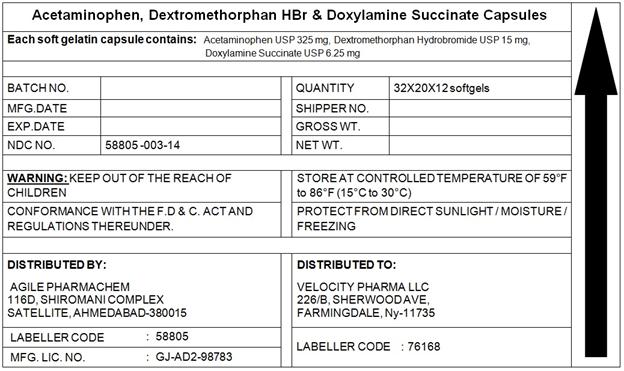
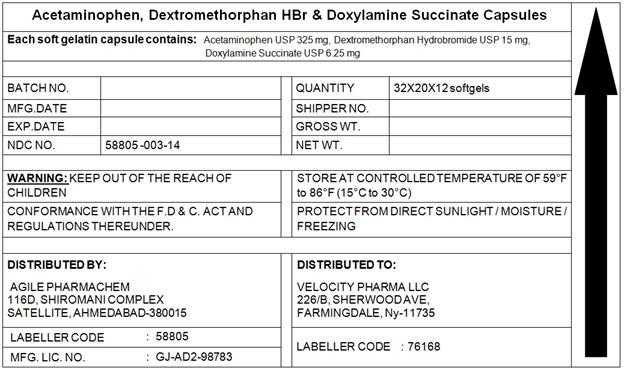
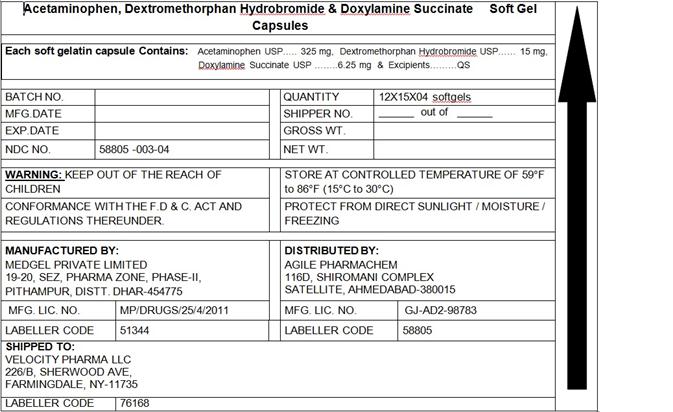
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ACETAMINOPHEN,DEXTROMETHORPHAN,DOXYLAMINE
acetaminophen,dextromethorphan,doxylamine capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:58805-003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 10 mg DOXYLAMINE SUCCINATE (UNII: V9BI9B5YI2) (DOXYLAMINE - UNII:95QB77JKPL) DOXYLAMINE SUCCINATE 6.25 mg Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) POLYETHYLENE GLYCOL 1000 (UNII: U076Q6Q621) POVIDONE (UNII: FZ989GH94E) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color GREEN Score no score Shape CAPSULE Size 22mm Flavor Imprint Code 215 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58805-003-14 7680 in 1 BLISTER PACK 05/01/2014 1 1 in 1 CARTON; Type 0: Not a Combination Product 2 NDC:58805-003-04 180 in 1 BLISTER PACK 05/01/2014 2 1 in 1 CARTON; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 05/01/2014 Labeler - Agile Pharmachem (650687853) Registrant - Agile Pharmachem (650687853)